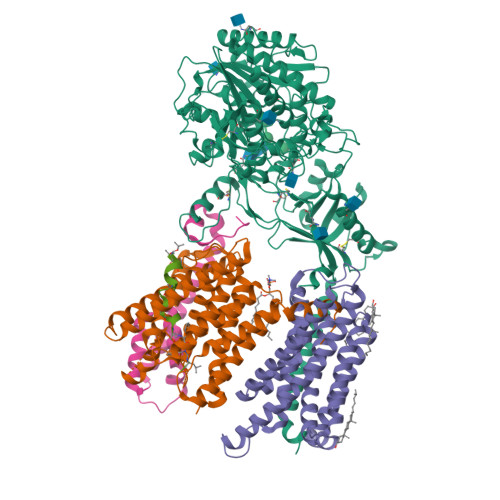
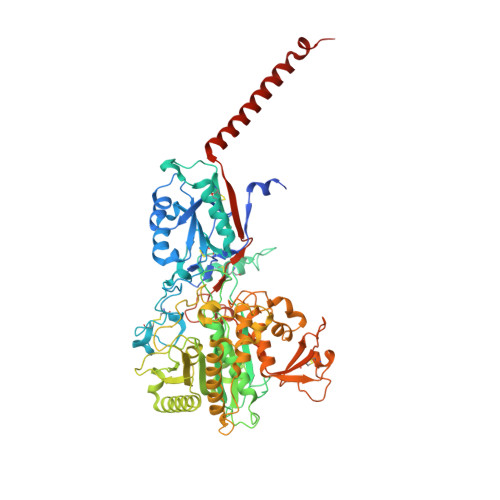
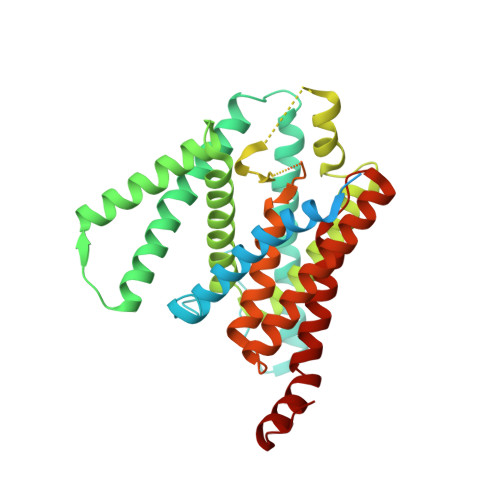
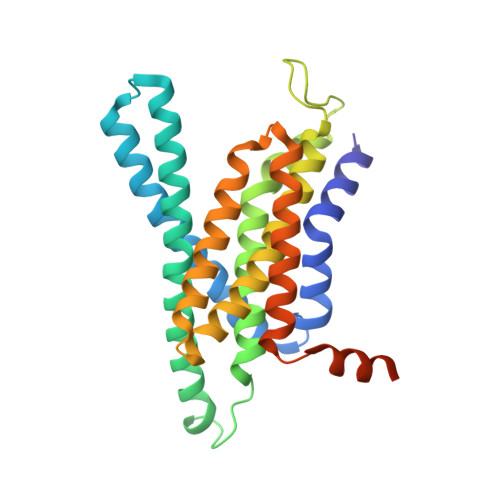
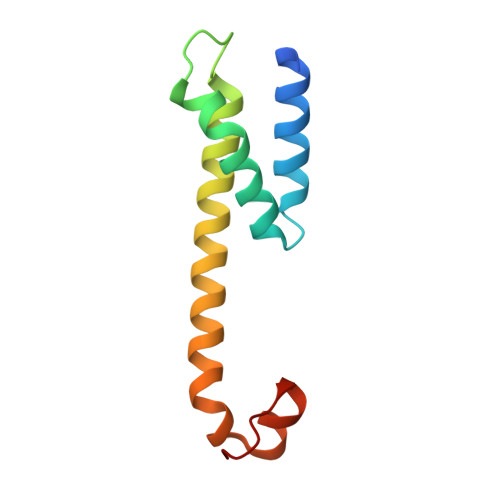
Familial Alzheimer mutations stabilize synaptotoxic gamma-secretase-substrate complexes.
Devkota, S., Zhou, R., Nagarajan, V., Maesako, M., Do, H., Noorani, A., Overmeyer, C., Bhattarai, S., Douglas, J.T., Saraf, A., Miao, Y., Ackley, B.D., Shi, Y., Wolfe, M.S.(2024) Cell Rep 43: 113761-113761
- PubMed: 38349793
- DOI: https://doi.org/10.1016/j.celrep.2024.113761
- Primary Citation of Related Structures:
8K8E - PubMed Abstract:
Mutations that cause familial Alzheimer's disease (FAD) are found in amyloid precursor protein (APP) and presenilin, the catalytic component of γ-secretase, that together produce amyloid β-peptide (Aβ). Nevertheless, whether Aβ is the primary disease driver remains controversial. We report here that FAD mutations disrupt initial proteolytic events in the multistep processing of APP substrate C99 by γ-secretase. Cryoelectron microscopy reveals that a substrate mimetic traps γ-secretase during the transition state, and this structure aligns with activated enzyme-substrate complex captured by molecular dynamics simulations. In silico simulations and in cellulo fluorescence microscopy support stabilization of enzyme-substrate complexes by FAD mutations. Neuronal expression of C99 and/or presenilin-1 in Caenorhabditis elegans leads to synaptic loss only with FAD-mutant transgenes. Designed mutations that stabilize the enzyme-substrate complex and block Aβ production likewise led to synaptic loss. Collectively, these findings implicate the stalled process-not the products-of γ-secretase cleavage of substrates in FAD pathogenesis.
Organizational Affiliation:
Department of Medicinal Chemistry, University of Kansas, Lawrence, KS, USA.