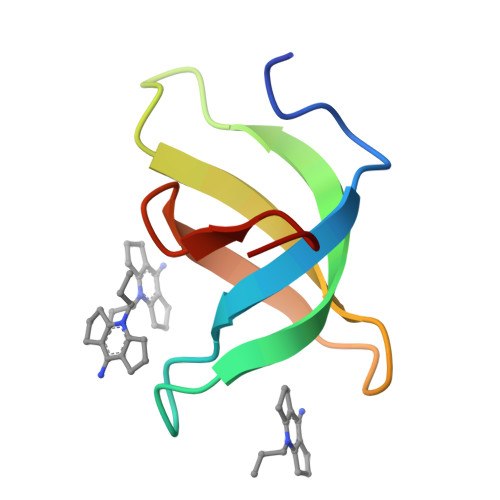
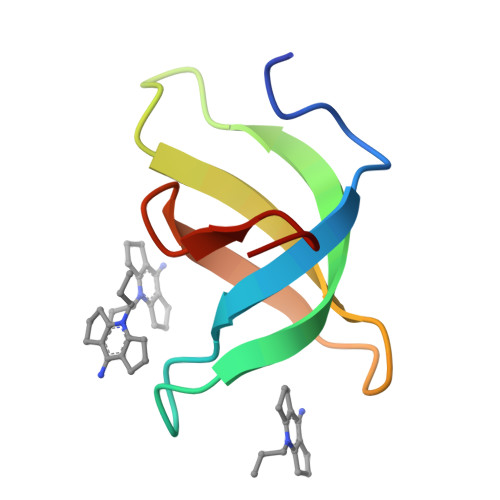
Crystal structure of Tudor domain of TDRD3 in complex with a small molecule antagonist.
Chen, M., Wang, Z., Li, W., Chen, Y., Xiao, Q., Shang, X., Huang, X., Wei, Z., Ji, X., Liu, Y.(2023) Biochim Biophys Acta Gene Regul Mech 1866: 194962-194962
- PubMed: 37499935
- DOI: https://doi.org/10.1016/j.bbagrm.2023.194962
- Primary Citation of Related Structures:
8JTN - PubMed Abstract:
Tudor domain-containing protein 3 (TDRD3) is involved in regulating transcription and translation, promoting breast cancer progression, and modulating neurodevelopment and mental health, making it a promising therapeutic target for associated diseases. The Tudor domain of TDRD3 is essential for its biological functions, and targeting this domain with potent and selective chemical probes may modulate its engagement with chromatin and related functions. Here we reported a study of TDRD3 antagonist following on our earlier work on the development of the SMN antagonist, Compound 1, and demonstrated that TDRD3 can bind effectively to Compound 2, a triple-ring analog of Compound 1. Our structural analysis suggested that the triple-ring compound bound better to TDRD3 due to its smaller side chain at Y566 compared to W102 in SMN. We also revealed that adding a small hydrophobic group to the N-methyl site of Compound 1 can improve binding. These findings provide a path for identifying antagonists for single canonical Tudor domain-containing proteins such as TDRD3 and SMN.
Organizational Affiliation:
Jiangsu Key Laboratory of Neuropsychiatric Diseases and College of Pharmaceutical Sciences, Jiangsu Province Engineering Research Center of Precision Diagnostics and Therapeutics Development, Soochow University, Suzhou, Jiangsu 215123, PR China.