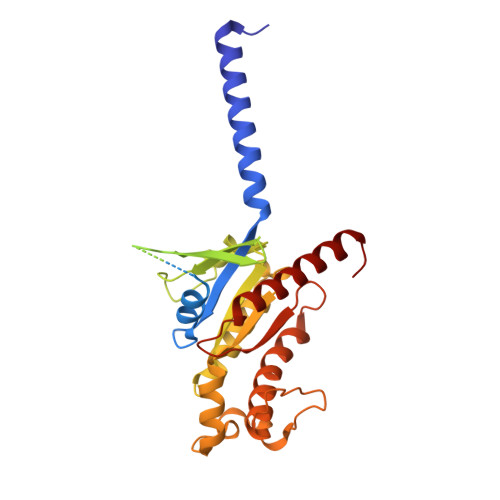
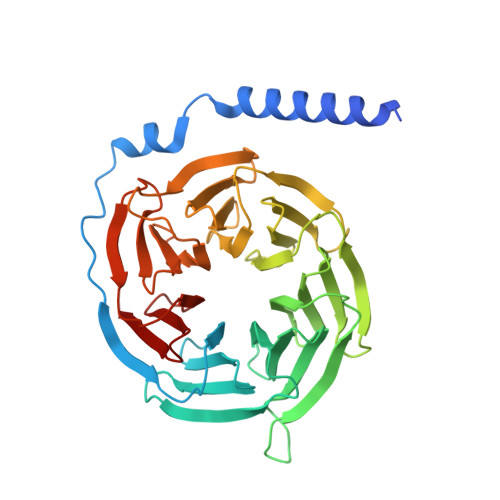
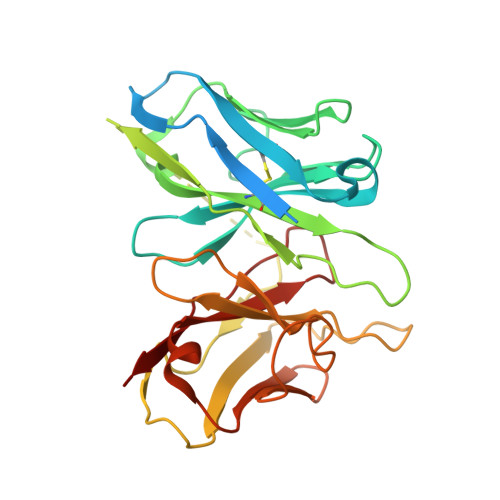
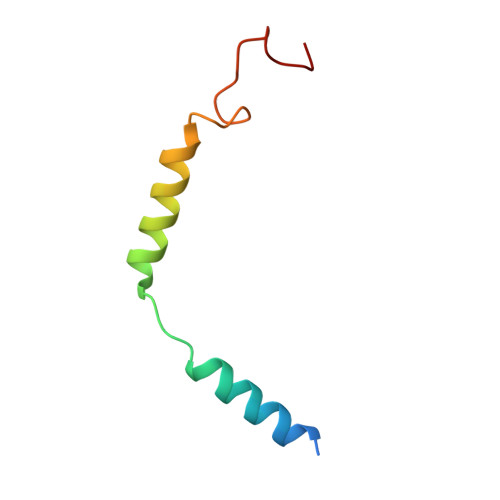
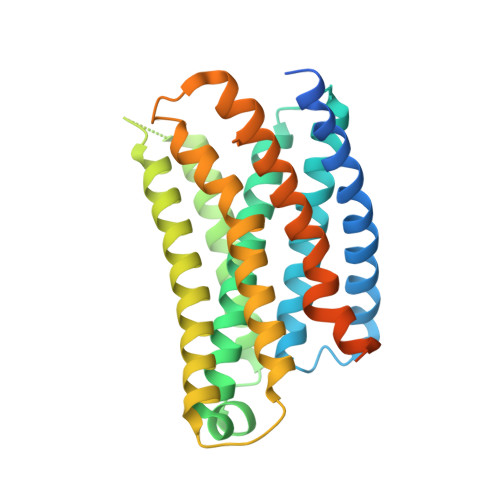
Ligand recognition and G protein coupling of the human itch receptor MRGPRX1.
Guo, L., Zhang, Y., Fang, G., Tie, L., Zhuang, Y., Xue, C., Liu, Q., Zhang, M., Zhu, K., You, C., Xu, P., Yuan, Q., Zhang, C., Liu, L., Rong, N., Peng, S., Liu, Y., Wang, C., Luo, X., Lv, Z., Kang, D., Yu, X., Zhang, C., Jiang, Y., Dong, X., Zhou, J., Liu, Z., Yang, F., Eric Xu, H., Sun, J.P.(2023) Nat Commun 14: 5004-5004
- PubMed: 37591889
- DOI: https://doi.org/10.1038/s41467-023-40705-z
- Primary Citation of Related Structures:
8JGB, 8JGF, 8JGG - PubMed Abstract:
MRGPRX1, a Mas-related GPCR (MRGPR), is a key receptor for itch perception and targeting MRGPRX1 may have potential to treat both chronic itch and pain. Here we report cryo-EM structures of the MRGPRX1-Gi1 and MRGPRX1-Gq trimers in complex with two peptide ligands, BAM8-22 and CNF-Tx2. These structures reveal a shallow orthosteric pocket and its conformational plasticity for sensing multiple different peptidic itch allergens. Distinct from MRGPRX2, MRGPRX1 contains a unique pocket feature at the extracellular ends of TM3 and TM4 to accommodate the peptide C-terminal "RF/RY" motif, which could serve as key mechanisms for peptidic allergen recognition. Below the ligand binding pocket, the G 6.48 XP 6.50 F 6.51 G 6.52 X (2) F/W 6.55 motif is essential for the inward tilting of the upper end of TM6 to induce receptor activation. Moreover, structural features inside the ligand pocket and on the cytoplasmic side of MRGPRX1 are identified as key elements for both Gi and Gq signaling. Collectively, our studies provide structural insights into understanding itch sensation, MRGPRX1 activation, and downstream G protein signaling.
Organizational Affiliation:
Advanced Medical Research Institute, Cheeloo College of Medicine, Shandong University, Jinan, China.