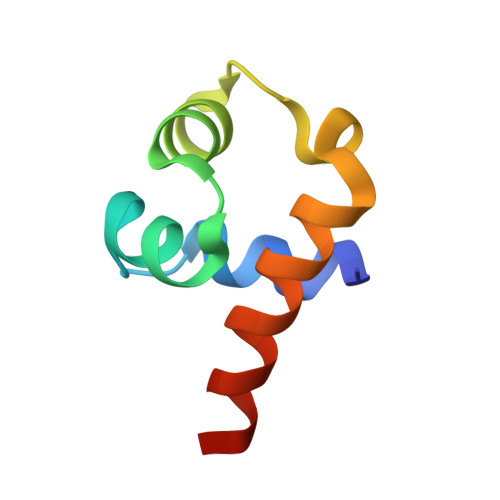
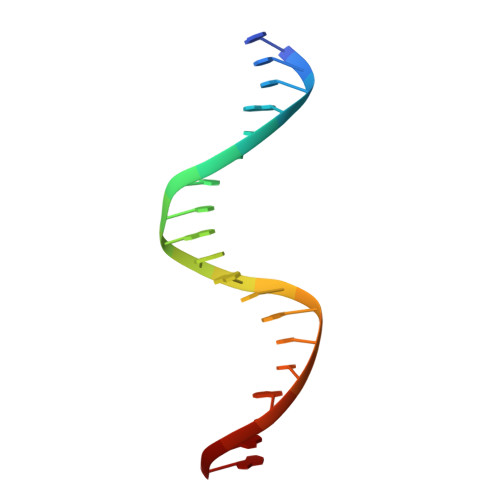
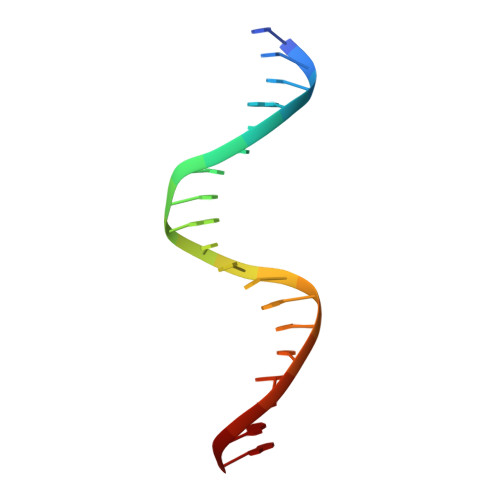
An anti-CRISPR that represses its own transcription while blocking Cas9-target DNA binding.
Deng, X., Sun, W., Li, X., Wang, J., Cheng, Z., Sheng, G., Wang, Y.(2024) Nat Commun 15: 1806-1806
- PubMed: 38418450
- DOI: https://doi.org/10.1038/s41467-024-45987-5
- Primary Citation of Related Structures:
8JFO, 8JFR, 8JFT, 8JFU, 8JG9 - PubMed Abstract:
AcrIIA15 is an anti-CRISPR (Acr) protein that inhibits Staphylococcus aureus Cas9 (SaCas9). Although previous studies suggested it has dual functions, the structural and biochemical basis for its two activities remains unclear. Here, we determined the cryo-EM structure of AcrIIA15 in complex with SaCas9-sgRNA to reveal the inhibitory mechanism of the Acr's C-terminal domain (CTD) in mimicking dsDNA to block protospacer adjacent motif (PAM) recognition. For the N-terminal domain (NTD), our crystal structures of the AcrIIA15-promoter DNA show that AcrIIA15 dimerizes through its NTD to recognize double-stranded (ds) DNA. Further, AcrIIA15 can simultaneously bind to both SaCas9-sgRNA and promoter DNA, creating a supercomplex of two Cas9s bound to two CTDs converging on a dimer of the NTD bound to a dsDNA. These findings shed light on AcrIIA15's inhibitory mechanisms and its autoregulation of transcription, enhancing our understanding of phage-host interactions and CRISPR defense.
Organizational Affiliation:
Key Laboratory of RNA Science and Engineering, Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China.