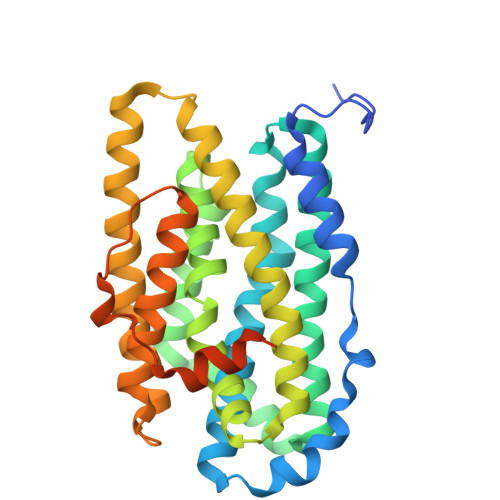
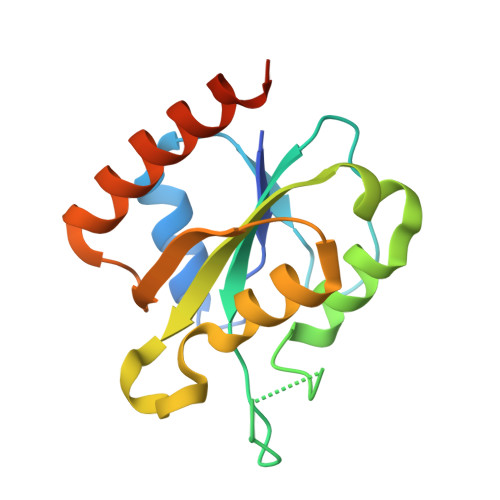
Structural insights into the initiation of free radical formation in the Class Ib ribonucleotide reductases in Mycobacteria.
Yadav, L.R., Sharma, V., Shanmugam, M., Mande, S.C.(2024) Curr Res Struct Biol 8: 100157-100157
- PubMed: 39399574
- DOI: https://doi.org/10.1016/j.crstbi.2024.100157
- Primary Citation of Related Structures:
8J4V, 8J4W, 8J4X, 8J4Y - PubMed Abstract:
Class I ribonucleotide reductases consisting of α and β subunits convert ribonucleoside diphosphates to deoxyribonucleoside diphosphates involving an intricate free radical mechanism. The generation of free radicals in the Class Ib ribonucleotide reductases is mediated by di-manganese ions in the β subunits and is externally assisted by flavodoxin-like NrdI subunit. This is unlike Class Ia ribonucleotide reductases, where the free radical generation is initiated at its di-iron centre in the β subunits with no external support from another subunit. Class 1b ribonucleotide reductase complex is an essential enzyme complex in the human pathogen Mycobacterium tuberculosis and its structural details are largely unknown. In this study we have determined the crystal structures of Mycobacterial NrdI in oxidised and reduced forms, and similarly those of NrdF2:NrdI complexes. These structures provide detailed atomic view of the mechanism of free radical generation in the β subunit in this pathogen. We observe a well-formed channel in NrdI from the surface leading to the buried FMN moiety and propose that oxygen molecule accesses FMN through it. The oxygen molecule is further converted to a superoxide ion upon electron transfer at the FMN moiety. Similarly, a path for superoxide radical transfer between NrdI and NrdF2 is also observed. The oxidation of Mn(II) in NrdF2I to high valent oxidation state (either Mn(III) or Mn(IV) assisted by the reduced FMN site was evidently confirmed by EPR studies. SEC-MALS and low resolution cryo-EM map indicate unusual stoichiometry of 2:1 in the M. tuberculosis NrdF2I complex. A density close to Tyr 110 at a distance <2.3 Å is observed, which we interpret as OH group. Overall, the study therefore provides important clues on the initiation of free radical generation in the β subunit of the ribonucleotide reductase complex in M. tuberculosis .
Organizational Affiliation:
National Centre for Cell Science, SPPU Campus, Ganeshkhind, Pune, 411007, India.