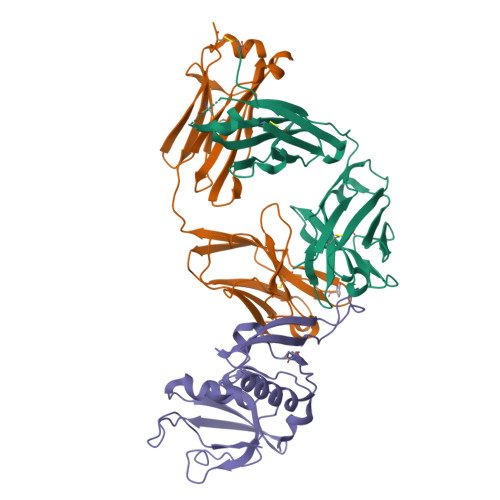
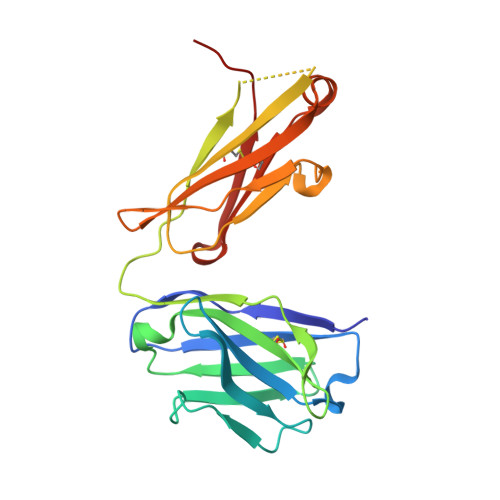
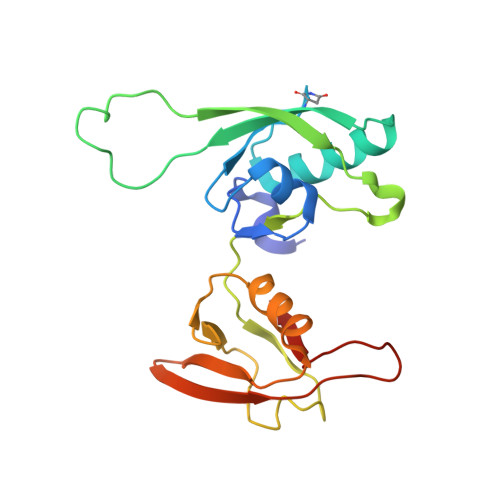
Recombinant production of antibody antigen-binding fragments with an N-terminal human growth hormone tag in mammalian cells.
Adachi, Y., Kaneko, M.K., Kato, Y., Nogi, T.(2023) Protein Expr Purif 208-209: 106289-106289
- PubMed: 37160213
- DOI: https://doi.org/10.1016/j.pep.2023.106289
- Primary Citation of Related Structures:
8IPC - PubMed Abstract:
Antigen-binding fragments (Fabs) of antibodies are both key biopharmaceuticals and valuable tools for basic life science. To streamline the production of diverse Fabs by capitalizing on standard and highly optimized protein production protocols, we here explore a method to prepare recombinant Fabs as secreted fusion proteins with an N-terminal human growth hormone domain and an octa-histidine tag. These tagged Fabs can be purified with standard immobilized metal chelate affinity chromatography. We first demonstrated Fab overproduction using the rat monoclonal antibody NZ-1. Optimization of linker residues enabled the complete removal of the tags by TEV protease, leaving only two additional residues at the N-terminus of the heavy chain. We purified NZ-1 Fab at ∼4 μg/mL of culture supernatant and further confirmed that the NZ-1 Fab from the fusion protein maintained its native fold and binding affinity for target cell-surface antigens. We also showed that several other Fabs of mouse IgG 1 s, the major subclass in mice, could be produced with the same procedure. Our preparation method can provide greater flexibility in functional and structural modifications of target Fabs because specialized purification techniques are not necessary.
Organizational Affiliation:
Graduate School of Medical Life Science, Yokohama City University, 1-7-29 Suehiro-cho, Tsurumi-ku, Yokohama, 230-0045, Japan.