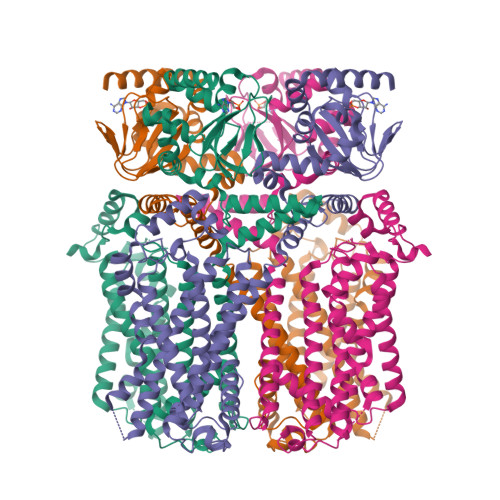
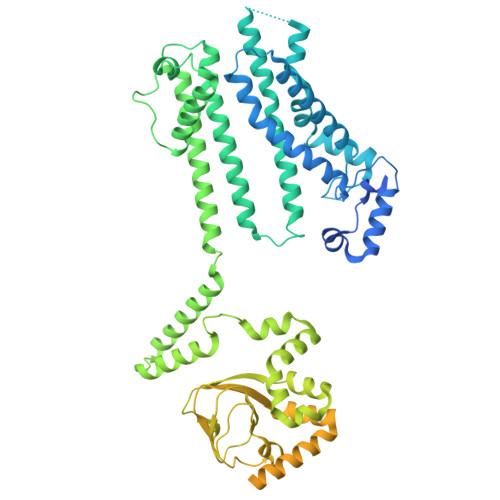
Cryo-EM structure of human HCN3 channel and its regulation by cAMP.
Yu, B., Lu, Q., Li, J., Cheng, X., Hu, H., Li, Y., Che, T., Hua, Y., Jiang, H., Zhang, Y., Xian, C., Yang, T., Fu, Y., Chen, Y., Nan, W., McCormick, P.J., Xiong, B., Duan, J., Zeng, B., Li, Y., Fu, Y., Zhang, J.(2024) J Biological Chem 300: 107288-107288
- PubMed: 38636662
- DOI: https://doi.org/10.1016/j.jbc.2024.107288
- Primary Citation of Related Structures:
8INZ, 8IO0 - PubMed Abstract:
HCN channels are important for regulating heart rhythm and nerve activity and have been studied as potential drug targets for treating depression, arrhythmia, nerve pain, and epilepsy. Despite possessing unique pharmacological properties, HCN channels share common characteristics in that they are activated by hyperpolarization and modulated by cAMP and other membrane lipids. However, the mechanisms of how these ligands bind and modulate HCN channels are unclear. In this study, we solved structures of full-length human HCN3 using cryo-EM and captured two different states, including a state without any ligand bound and a state with cAMP bound. Our structures reveal the novel binding sites for cholesteryl hemisuccinate in apo state and show how cholesteryl hemisuccinate and cAMP binding cause conformational changes in different states. These findings explain how these small modulators are sensed in mammals at the molecular level. The results of our study could help to design more potent and specific compounds to influence HCN channel activity and offer new therapeutic possibilities for diseases that lack effective treatment.
Organizational Affiliation:
The MOE Basic Research and Innovation Center for the Targeted Therapeutics of Solid Tumors, School of Basic Medical Sciences, Jiangxi Medical College, Nanchang University, Nanchang, China; The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China.