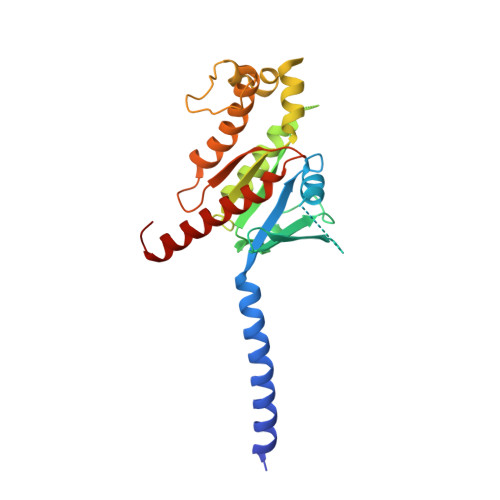
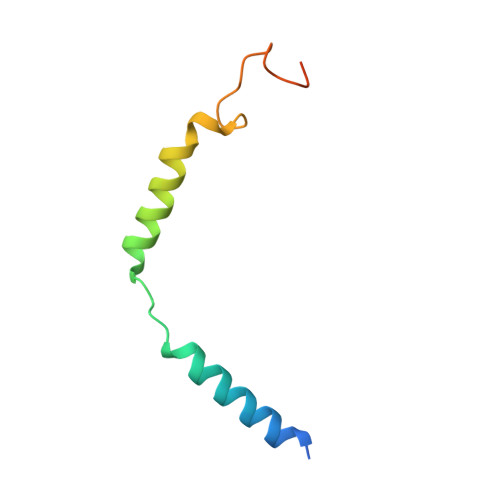
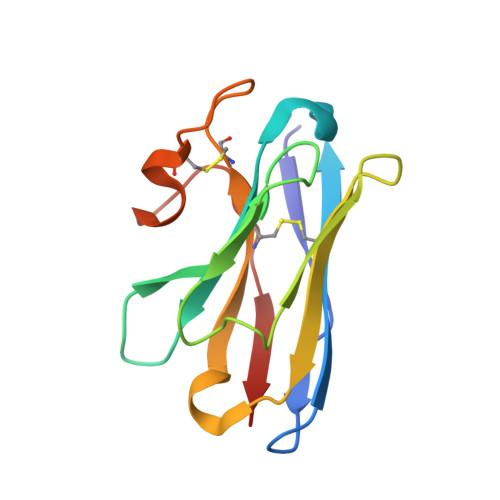
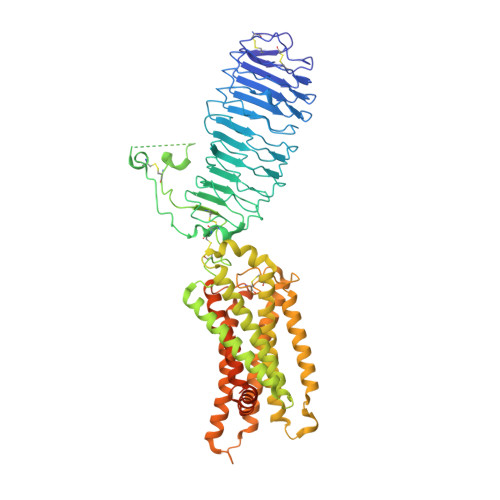
Mechanism of hormone and allosteric agonist mediated activation of follicle stimulating hormone receptor.
Duan, J., Xu, P., Zhang, H., Luan, X., Yang, J., He, X., Mao, C., Shen, D.D., Ji, Y., Cheng, X., Jiang, H., Jiang, Y., Zhang, S., Zhang, Y., Xu, H.E.(2023) Nat Commun 14: 519-519
- PubMed: 36720854
- DOI: https://doi.org/10.1038/s41467-023-36170-3
- Primary Citation of Related Structures:
8I2G, 8I2H - PubMed Abstract:
Follicle stimulating hormone (FSH) is an essential glycoprotein hormone for human reproduction, which functions are mediated by a G protein-coupled receptor, FSHR. Aberrant FSH-FSHR signaling causes infertility and ovarian hyperstimulation syndrome. Here we report cryo-EM structures of FSHR in both inactive and active states, with the active structure bound to FSH and an allosteric agonist compound 21 f. The structures of FSHR are similar to other glycoprotein hormone receptors, highlighting a conserved activation mechanism of hormone-induced receptor activation. Compound 21 f formed extensive interactions with the TMD to directly activate FSHR. Importantly, the unique residue H615 7.42 in FSHR plays an essential role in determining FSHR selectivity for various allosteric agonists. Together, our structures provide a molecular basis of FSH and small allosteric agonist-mediated FSHR activation, which could inspire the design of FSHR-targeted drugs for the treatment of infertility and controlled ovarian stimulation for in vitro fertilization.
Organizational Affiliation:
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 201203, Shanghai, China.