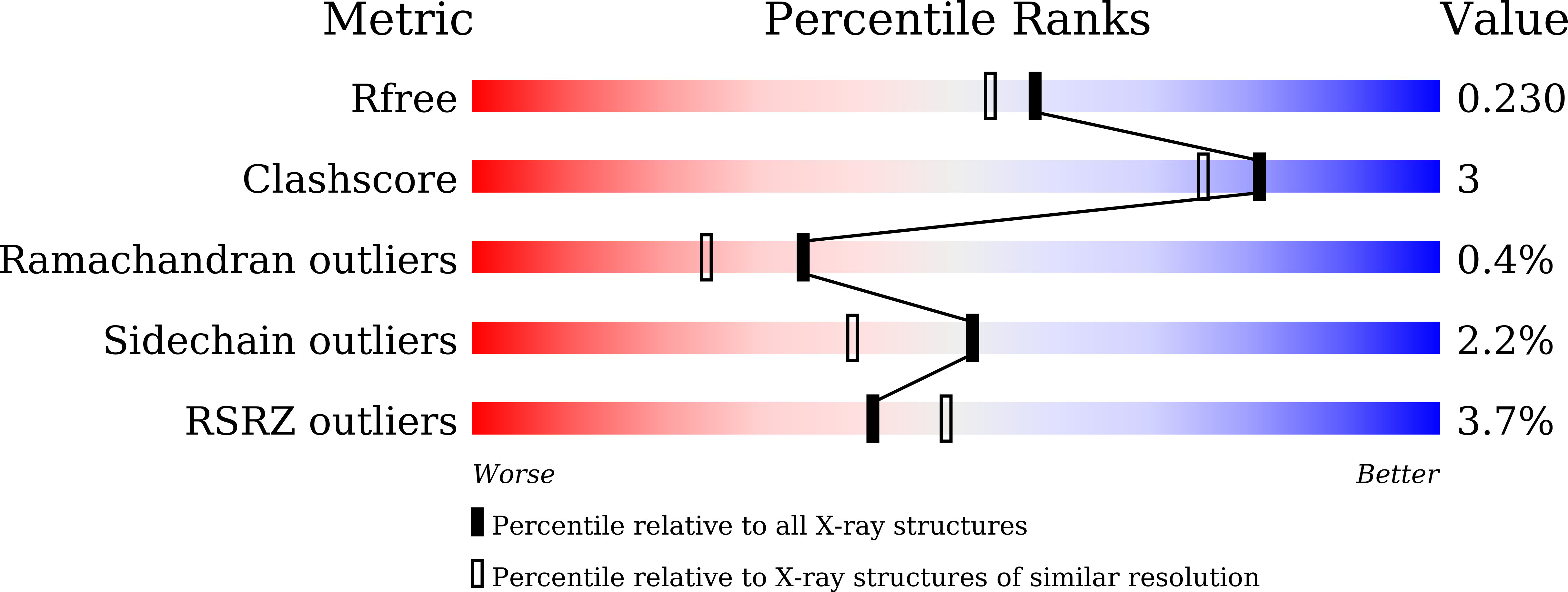
Discovery of 2-Aminothiazole-4-carboxylic Acids as Broad-Spectrum Metallo-beta-lactamase Inhibitors by Mimicking Carbapenem Hydrolysate Binding.
Yan, Y.H., Zhang, T.T., Li, R., Wang, S.Y., Wei, L.L., Wang, X.Y., Zhu, K.R., Li, S.R., Liang, G.Q., Yang, Z.B., Yang, L.L., Qin, S., Li, G.B.(2023) J Med Chem 66: 13746-13767
- PubMed: 37791640
- DOI: https://doi.org/10.1021/acs.jmedchem.3c01189
- Primary Citation of Related Structures:
8HX5, 8HXE, 8HXI, 8HXN, 8HXO, 8HXP, 8HXU, 8HXV, 8HXW, 8HY1, 8HY2, 8HY6, 8HYD, 8JAO - PubMed Abstract:
Metallo-β-lactamases (MBLs) are zinc-dependent enzymes capable of hydrolyzing all bicyclic β-lactam antibiotics, posing a great threat to public health. However, there are currently no clinically approved MBL inhibitors. Despite variations in their active sites, MBLs share a common catalytic mechanism with carbapenems, forming similar reaction species and hydrolysates. We here report the development of 2-aminothiazole-4-carboxylic acids (AtCs) as broad-spectrum MBL inhibitors by mimicking the anchor pharmacophore features of carbapenem hydrolysate binding. Several AtCs manifested potent activity against B1, B2, and B3 MBLs. Crystallographic analyses revealed a common binding mode of AtCs with B1, B2, and B3 MBLs, resembling binding observed in the MBL-carbapenem product complexes. AtCs restored Meropenem activity against MBL-producing isolates. In the murine sepsis model, AtCs exhibited favorable synergistic efficacy with Meropenem, along with acceptable pharmacokinetics and safety profiles. This work offers promising lead compounds and a structural basis for the development of potential drug candidates to combat MBL-mediated antimicrobial resistance.
Organizational Affiliation:
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu 610041, China.