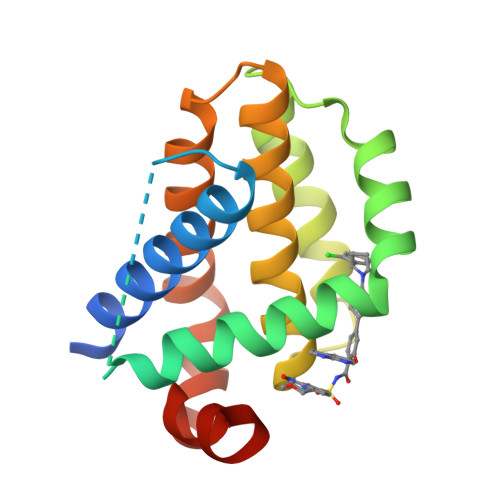
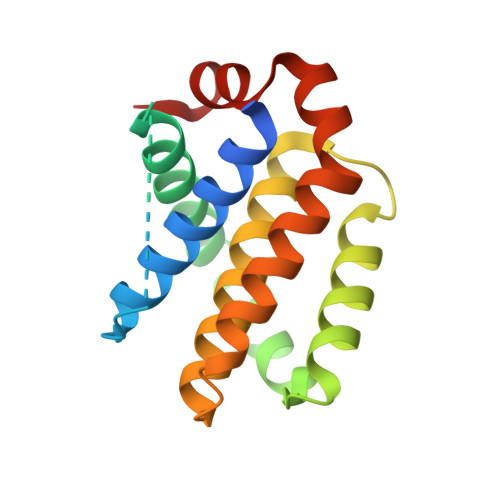
Discovery of the Clinical Candidate Sonrotoclax (BGB-11417), a Highly Potent and Selective Inhibitor for Both WT and G101V Mutant Bcl-2.
Guo, Y., Xue, H., Hu, N., Liu, Y., Sun, H., Yu, D., Qin, L., Shi, G., Wang, F., Xin, L., Sun, W., Zhang, F., Song, X., Li, S., Wei, Q., Guo, Y., Li, Y., Liu, X., Chen, S., Zhang, T., Wu, Y., Su, D., Zhu, Y., Xu, A., Xu, H., Yang, S., Zheng, Z., Liu, J., Yang, X., Yuan, X., Hong, Y., Sun, X., Guo, Y., Zhou, C., Liu, X., Wang, L., Wang, Z.(2024) J Med Chem 67: 7836-7858
- PubMed: 38695063
- DOI: https://doi.org/10.1021/acs.jmedchem.4c00027
- Primary Citation of Related Structures:
8HTR, 8HTS - PubMed Abstract:
The approval of venetoclax, a B-cell lymphoma-2 (Bcl-2) selective inhibitor, for the treatment of chronic lymphocytic leukemia demonstrated that the antiapoptotic protein Bcl-2 is a druggable target for B-cell malignancies. However, venetoclax's limited potency cannot produce a strong, durable clinical benefit in other Bcl-2-mediated malignancies (e.g., diffuse large B-cell lymphomas) and multiple recurrent Bcl-2 mutations (e.g., G101V) have been reported to mediate resistance to venetoclax after long-term treatment. Herein, we described novel Bcl-2 inhibitors with increased potency for both wild-type (WT) and mutant Bcl-2. Comprehensive structure optimization led to the clinical candidate BGB-11417 (compound 12e , sonrotoclax), which exhibits strong in vitro and in vivo inhibitory activity against both WT Bcl-2 and the G101V mutant, as well as excellent selectivity over Bcl-x L without obvious cytochrome P450 inhibition. Currently, BGB-11417 is undergoing phase II/III clinical assessments as monotherapy and combination treatment.