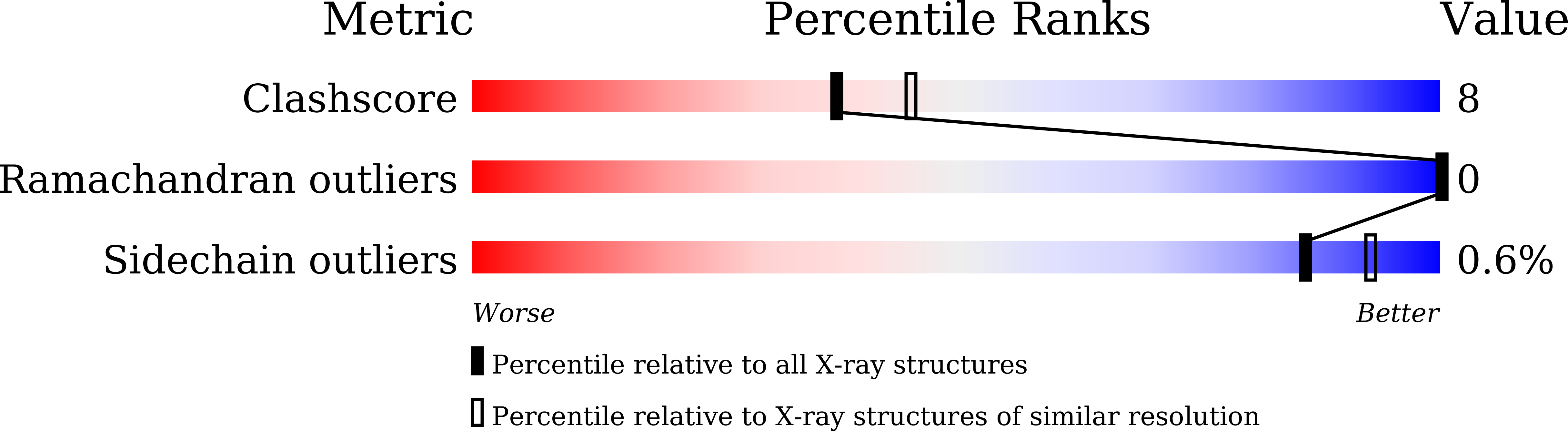
Structural characterization on a beta-agarase Aga86A_Wa from Wenyingzhuangia aestuarii reveals the prevalent methyl-galactose accommodation capacity of GH86 enzymes at subsite -1.
Zhang, Y., Dong, S., Chen, G., Cao, S., Shen, J., Mei, X., Cui, Q., Feng, Y., Chang, Y., Wang, Y., Xue, C.(2023) Carbohydr Polym 306: 120594-120594
- PubMed: 36746585
- DOI: https://doi.org/10.1016/j.carbpol.2023.120594
- Primary Citation of Related Structures:
8H97 - PubMed Abstract:
Agarans are sulfated galactans extracted from red algae with high structural complexity, of which natural methylation often occurs on the O-6 position of its β-d-galactopyranose units. Although many agaran degrading enzymes, including agarases and porphyranases, have been characterized, little attention has been paid to the tolerance of methyl groups at cleavage subsites. In this study, the structure of GH86 β-agarase Aga86A_Wa from Wenyingzhuangia aestuarii was determined by X-ray crystallography and investigated from a structural biology perspective. The structure indicated that an accommodation pocket formed by F367, Y280, and Q326 at subsite -1 contributes to the methyl-galactose tolerance of Aga86A_Wa. Furthermore, we found that similar accommodation pockets were present in the structures of two other GH86 enzymes BuGH86 from Bacteroides uniformis and BpGH86A from Phocaeicola plebeius, and their previously undisclosed methyl-galactose tolerance was verified, validating the function of the pockets. Phylogenetic analysis, structural modeling, and hydrolysis product characterization suggested that the methyl-galactose accommodation capacity at subsite -1 was prevalent in GH86 members. These findings achieve a better understanding of the function and mechanism of GH86 agaran degrading enzymes, and will facilitate the precise preparation of agaran oligosaccharides by employing defined tools.
Organizational Affiliation:
College of Food Science and Engineering, Ocean University of China, Qingdao 266003, PR China.