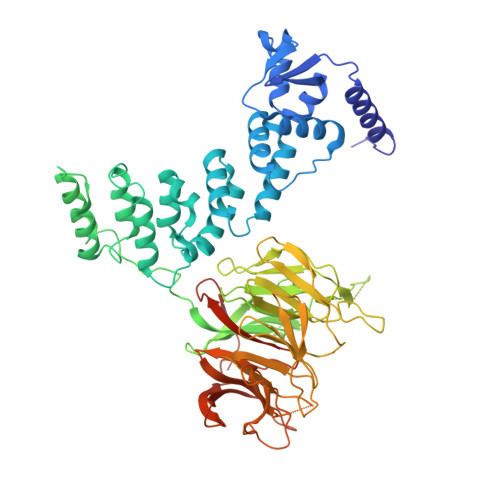
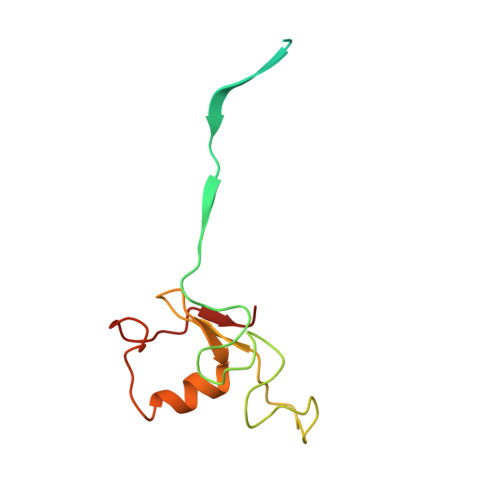
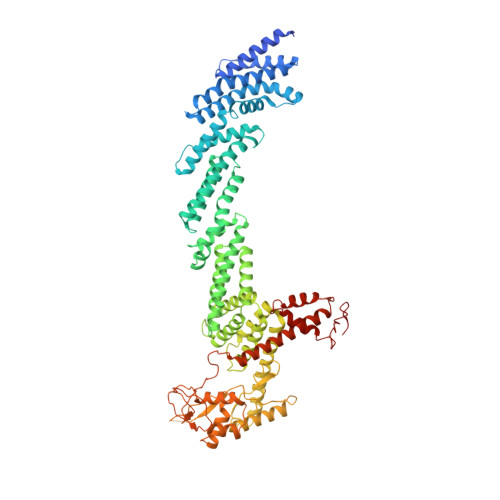
Dynamic molecular architecture and substrate recruitment of cullin3-RING E3 ligase CRL3 KBTBD2.
Hu, Y., Zhang, Z., Mao, Q., Zhang, X., Hao, A., Xun, Y., Wang, Y., Han, L., Zhan, W., Liu, Q., Yin, Y., Peng, C., Moresco, E.M.Y., Chen, Z., Beutler, B., Sun, L.(2024) Nat Struct Mol Biol 31: 336-350
- PubMed: 38332366
- DOI: https://doi.org/10.1038/s41594-023-01182-6
- Primary Citation of Related Structures:
8GQ6, 8H33, 8H34, 8H35, 8H36, 8H37, 8H38, 8H3A, 8H3F, 8H3Q, 8H3R - PubMed Abstract:
Phosphatidylinositol 3-kinase α, a heterodimer of catalytic p110α and one of five regulatory subunits, mediates insulin- and insulin like growth factor-signaling and, frequently, oncogenesis. Cellular levels of the regulatory p85α subunit are tightly controlled by regulated proteasomal degradation. In adipose tissue and growth plates, failure of K48-linked p85α ubiquitination causes diabetes, lipodystrophy and dwarfism in mice, as in humans with SHORT syndrome. Here we elucidated the structures of the key ubiquitin ligase complexes regulating p85α availability. Specificity is provided by the substrate receptor KBTBD2, which recruits p85α to the cullin3-RING E3 ubiquitin ligase (CRL3). CRL3 KBTBD2 forms multimers, which disassemble into dimers upon substrate binding (CRL3 KBTBD2 -p85α) and/or neddylation by the activator NEDD8 (CRL3 KBTBD2 ~N8), leading to p85α ubiquitination and degradation. Deactivation involves dissociation of NEDD8 mediated by the COP9 signalosome and displacement of KBTBD2 by the inhibitor CAND1. The hereby identified structural basis of p85α regulation opens the way to better understanding disturbances of glucose regulation, growth and cancer.
Organizational Affiliation:
Shanghai Fifth People's Hospital, Shanghai Institute of Infectious Disease and Biosecurity, Shanghai Key Laboratory of Medical Epigenetics and Institutes of Biomedical Sciences, Fudan University, Shanghai, China.