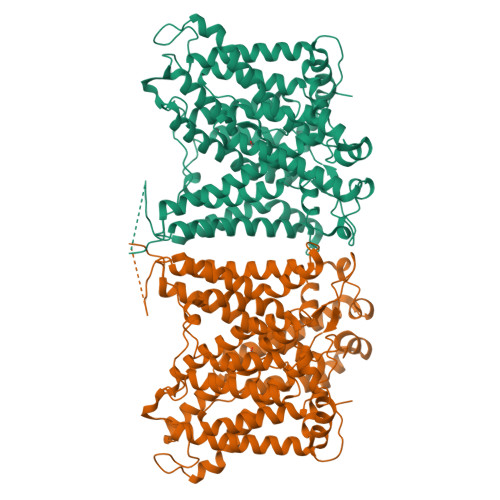
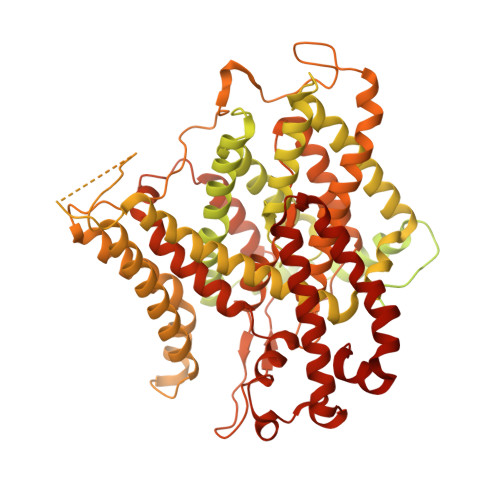
The structural basis of the pH-homeostasis mediated by the Cl - /HCO 3 - exchanger, AE2.
Zhang, Q., Jian, L., Yao, D., Rao, B., Xia, Y., Hu, K., Li, S., Shen, Y., Cao, M., Qin, A., Zhao, J., Cao, Y.(2023) Nat Commun 14: 1812-1812
- PubMed: 37002221
- DOI: https://doi.org/10.1038/s41467-023-37557-y
- Primary Citation of Related Structures:
8GV8, 8GV9, 8GVA, 8GVC, 8GVE, 8GVF, 8GVH - PubMed Abstract:
The cell maintains its intracellular pH in a narrow physiological range and disrupting the pH-homeostasis could cause dysfunctional metabolic states. Anion exchanger 2 (AE2) works at high cellular pH to catalyze the exchange between the intracellular HCO 3 - and extracellular Cl - , thereby maintaining the pH-homeostasis. Here, we determine the cryo-EM structures of human AE2 in five major operating states and one transitional hybrid state. Among those states, the AE2 shows the inward-facing, outward-facing, and intermediate conformations, as well as the substrate-binding pockets at two sides of the cell membrane. Furthermore, critical structural features were identified showing an interlock mechanism for interactions among the cytoplasmic N-terminal domain and the transmembrane domain and the self-inhibitory effect of the C-terminal loop. The structural and cell-based functional assay collectively demonstrate the dynamic process of the anion exchange across membranes and provide the structural basis for the pH-sensitive pH-rebalancing activity of AE2.
Organizational Affiliation:
Department of Orthopaedics, Shanghai Key Laboratory of Orthopaedic Implant, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, 200011, Shanghai, China.