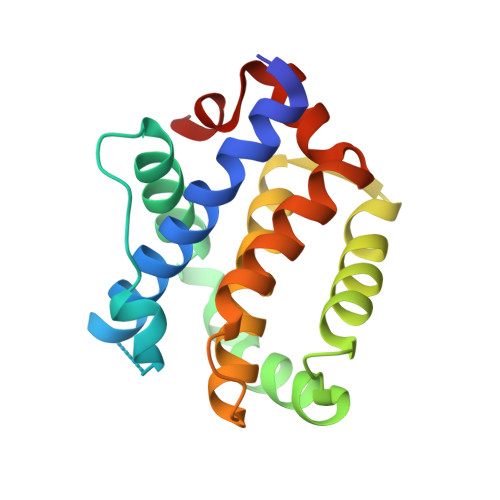
Structural basis for proapoptotic activation of Bak by the noncanonical BH3-only protein Pxt1.
Lim, D., Choe, S.H., Jin, S., Lee, S., Kim, Y., Shin, H.C., Choi, J.S., Oh, D.B., Kim, S.J., Seo, J., Ku, B.(2023) PLoS Biol 21: e3002156-e3002156
- PubMed: 37315086
- DOI: https://doi.org/10.1371/journal.pbio.3002156
- Primary Citation of Related Structures:
8GSV - PubMed Abstract:
Bak is a critical executor of apoptosis belonging to the Bcl-2 protein family. Bak contains a hydrophobic groove where the BH3 domain of proapoptotic Bcl-2 family members can be accommodated, which initiates its activation. Once activated, Bak undergoes a conformational change to oligomerize, which leads to mitochondrial destabilization and the release of cytochrome c into the cytosol and eventual apoptotic cell death. In this study, we investigated the molecular aspects and functional consequences of the interaction between Bak and peroxisomal testis-specific 1 (Pxt1), a noncanonical BH3-only protein exclusively expressed in the testis. Together with various biochemical approaches, this interaction was verified and analyzed at the atomic level by determining the crystal structure of the Bak-Pxt1 BH3 complex. In-depth biochemical and cellular analyses demonstrated that Pxt1 functions as a Bak-activating proapoptotic factor, and its BH3 domain, which mediates direct intermolecular interaction with Bak, plays a critical role in triggering apoptosis. Therefore, this study provides a molecular basis for the Pxt1-mediated novel pathway for the activation of apoptosis and expands our understanding of the cell death signaling coordinated by diverse BH3 domain-containing proteins.
Organizational Affiliation:
Disease Target Structure Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea.