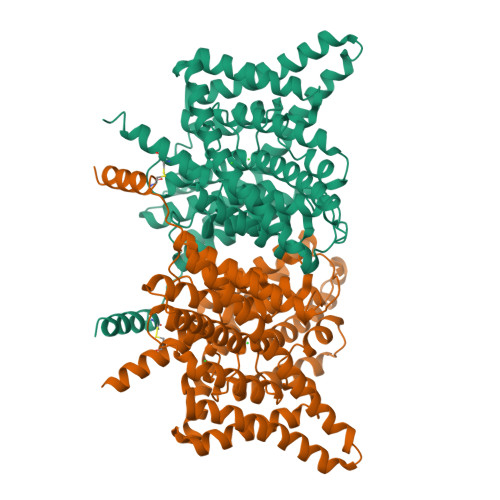
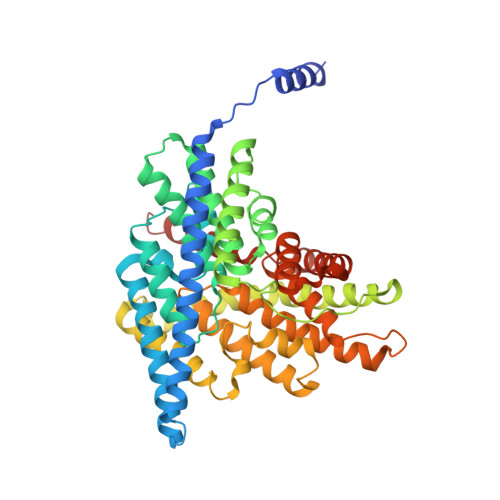
Structural basis of pH-dependent activation in a CLC transporter.
Fortea, E., Lee, S., Chadda, R., Argyros, Y., Sandal, P., Mahoney-Kruszka, R., Ciftci, H.D., Falzone, M.E., Huysmans, G., Robertson, J.L., Boudker, O., Accardi, A.(2024) Nat Struct Mol Biol 31: 644-656
- PubMed: 38279055
- DOI: https://doi.org/10.1038/s41594-023-01210-5
- Primary Citation of Related Structures:
8GA0, 8GA1, 8GA3, 8GA5, 8GAH - PubMed Abstract:
CLCs are dimeric chloride channels and anion/proton exchangers that regulate processes such as muscle contraction and endo-lysosome acidification. Common gating controls their activity; its closure simultaneously silences both protomers, and its opening allows them to independently transport ions. Mutations affecting common gating in human CLCs cause dominant genetic disorders. The structural rearrangements underlying common gating are unknown. Here, using single-particle cryo-electron microscopy, we show that the prototypical Escherichia coli CLC-ec1 undergoes large-scale rearrangements in activating conditions. The slow, pH-dependent remodeling of the dimer interface leads to the concerted opening of the intracellular H + pathways and is required for transport. The more frequent formation of short water wires in the open H + pathway enables Cl - pore openings. Mutations at disease-causing sites favor CLC-ec1 activation and accelerate common gate opening in the human CLC-7 exchanger. We suggest that the pH activation mechanism of CLC-ec1 is related to the common gating of CLC-7.
Organizational Affiliation:
Department of Physiology and Biophysics, Weill Cornell Medical School, New York, NY, USA.