Exploring Covalent Bond Formation at Tyr-82 for Inhibition of Ral GTPase Activation.
Landgraf, A.D., Yeh, I.J., Ghozayel, M.K., Bum-Erdene, K., Gonzalez-Gutierrez, G., Meroueh, S.O.(2023) ChemMedChem 18: e202300272-e202300272
- PubMed: 37269475
- DOI: https://doi.org/10.1002/cmdc.202300272
- Primary Citation of Related Structures:
8FJH, 8FJI - PubMed Abstract:
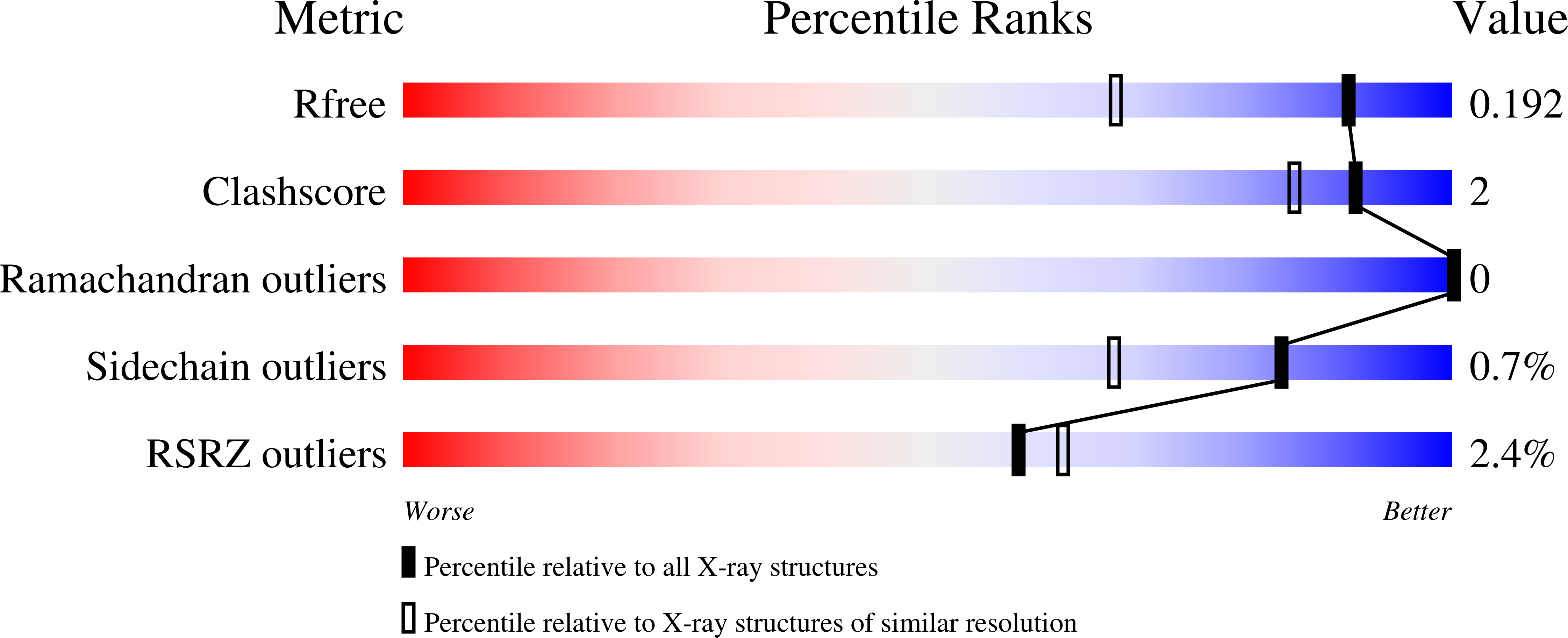
Ral RAS GTPases are directly activated by KRAS through a trimeric complex with a guanine exchange factor. Ral is considered undruggable and lacks an accessible cysteine for covalent drug development. Previously we had reported an aryl sulfonyl fluoride fragment that formed a covalent bond at Tyr-82 on Ral and created a deep and well-defined pocket. Here, we explore this pocket further through design and synthesis of several fragment derivatives. The fragment core is modified by introducing tetrahydronaphthalene or benzodioxane rings to enhance affinity and stability of the sulfonyl fluoride reactive group. The deep pocket in the Switch II region is also explored by modifying the aromatic ring of the fragment that is ensconced into the pocket. Compounds 19 (SOF-658) and 26 (SOF-648) formed a single robust adduct specifically at Tyr-82, inhibited Ral GTPase exchange in buffer and in mammalian cells, and blocked invasion of pancreatic ductal adenocarcinoma cancer cells. Compound 19 (SOF-658) was stable in buffer, mouse, and human microsomes suggesting that further optimization could lead to small molecules to probe Ral activity in tumor models.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46202, USA.