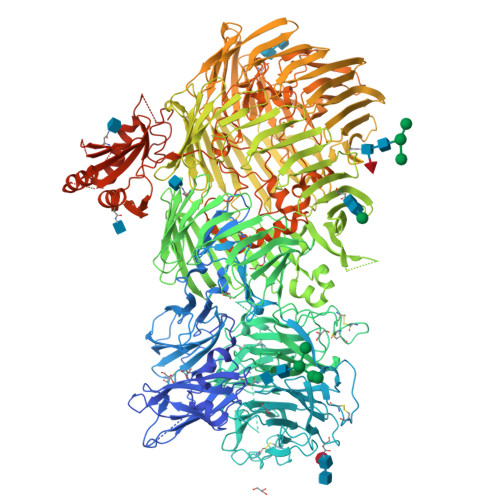
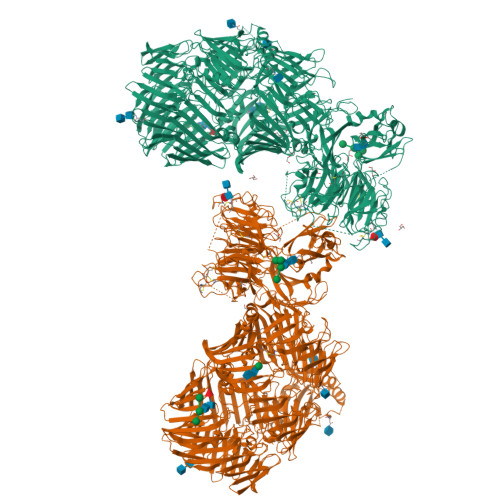
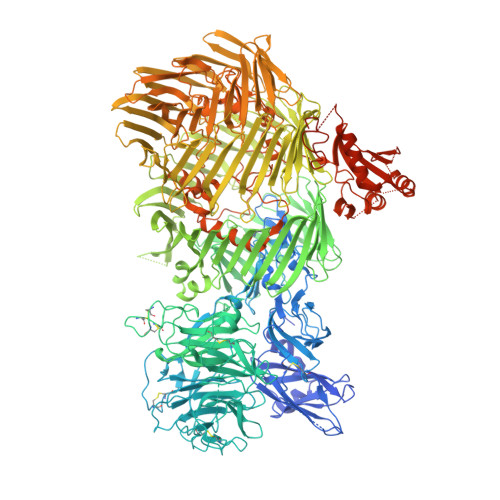
The structure of fly Teneurin-m reveals an asymmetric self-assembly that allows expansion into zippers.
Li, J., Bandekar, S.J., Arac, D.(2023) EMBO Rep 24: e56728-e56728
- PubMed: 37165720
- DOI: https://doi.org/10.15252/embr.202256728
- Primary Citation of Related Structures:
8FIA - PubMed Abstract:
Teneurins are conserved cell adhesion molecules essential for embryogenesis and neural development in animals. Key to teneurin function is the ability of its extracellular region to form homophilic interactions in cis and/or in trans. However, our molecular understanding of teneurin homophilic interaction remains largely incomplete. Here, we showed that an extracellular fragment of Teneurin-m, the major teneurin homolog in flies, behaves as a homodimer in solution. The structure of Teneurin-m revealed that the transthyretin-related domain from one protomer and the β-propeller domain from the other mediates Teneurin-m self-association, which is abolished by point mutation of conserved residues. Strikingly, this architecture generates an asymmetric oligomerization interface that enables expansion of Teneurin-m into long zipper arrays reminiscent of protocadherins. An alternatively spliced site that exists only in vertebrates and regulates homophilic interaction in mammalian teneurins overlaps with the fly Teneurin-m self-association interface. Our work provides a molecular understanding of teneurin homophilic interaction and sheds light on its role in teneurin function throughout evolution.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Chicago, Chicago, IL, USA.