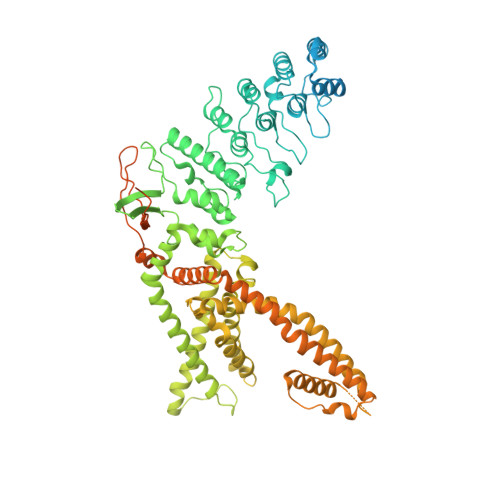
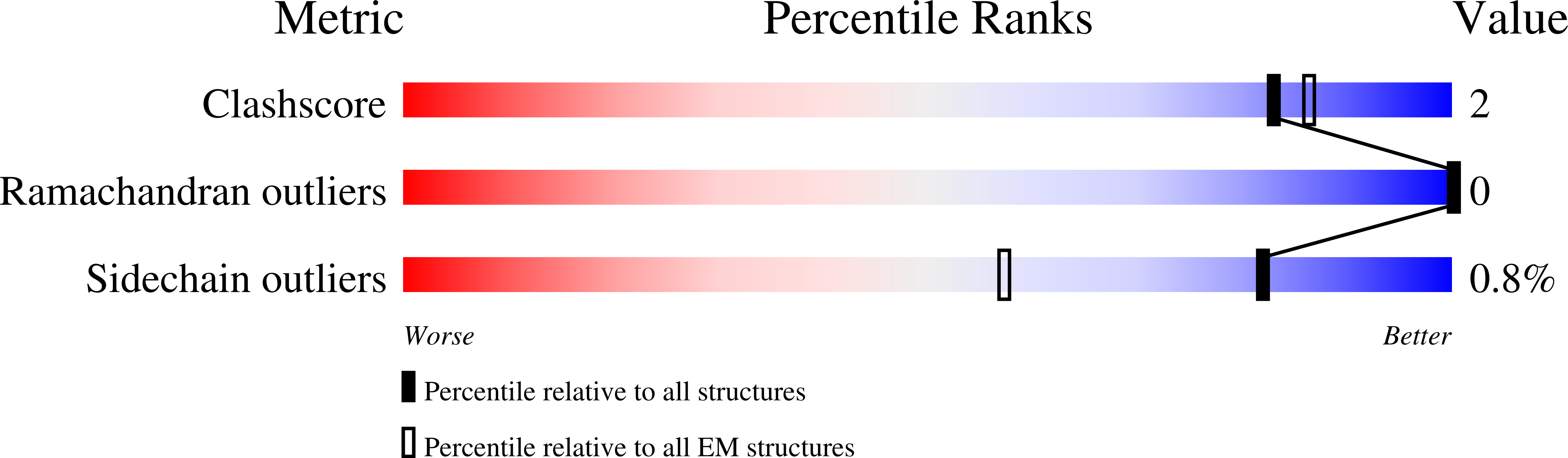TRPV4-Rho GTPase complex structures reveal mechanisms of gating and disease.
Kwon, D.H., Zhang, F., McCray, B.A., Feng, S., Kumar, M., Sullivan, J.M., Im, W., Sumner, C.J., Lee, S.Y.(2023) Nat Commun 14: 3732-3732
- PubMed: 37353484
- DOI: https://doi.org/10.1038/s41467-023-39345-0
- Primary Citation of Related Structures:
8FC7, 8FC8, 8FC9, 8FCA, 8FCB - PubMed Abstract:
Crosstalk between ion channels and small GTPases is critical during homeostasis and disease, but little is known about the structural underpinnings of these interactions. TRPV4 is a polymodal, calcium-permeable cation channel that has emerged as a potential therapeutic target in multiple conditions. Gain-of-function mutations also cause hereditary neuromuscular disease. Here, we present cryo-EM structures of human TRPV4 in complex with RhoA in the ligand-free, antagonist-bound closed, and agonist-bound open states. These structures reveal the mechanism of ligand-dependent TRPV4 gating. Channel activation is associated with rigid-body rotation of the intracellular ankyrin repeat domain, but state-dependent interaction with membrane-anchored RhoA constrains this movement. Notably, many residues at the TRPV4-RhoA interface are mutated in disease and perturbing this interface by introducing mutations into either TRPV4 or RhoA increases TRPV4 channel activity. Together, these results suggest that RhoA serves as an auxiliary subunit for TRPV4, regulating TRPV4-mediated calcium homeostasis and disruption of TRPV4-RhoA interactions can lead to TRPV4-related neuromuscular disease. These insights will help facilitate TRPV4 therapeutics development.
Organizational Affiliation:
Department of Biochemistry, Duke University School of Medicine, Durham, NC, 27710, USA.