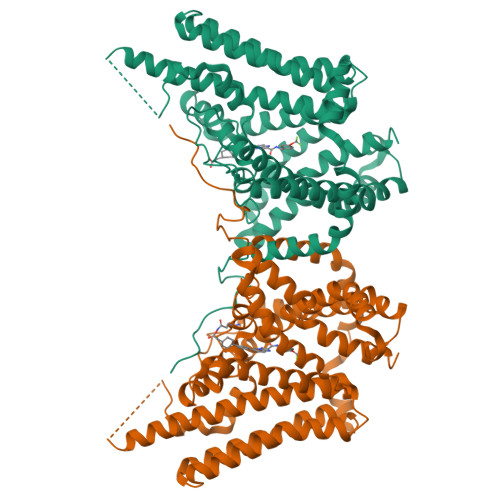
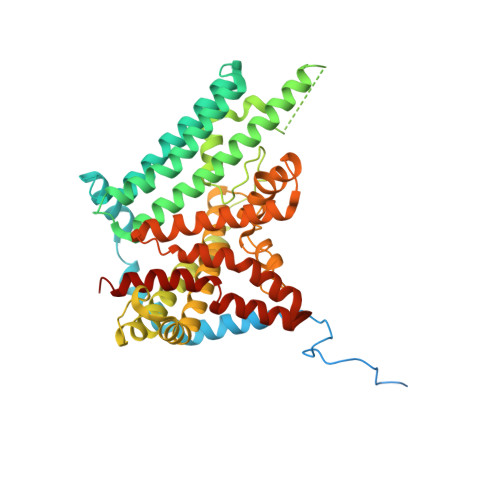
Mechanism of action for small-molecule inhibitors of triacylglycerol synthesis.
Sui, X., Wang, K., Song, K., Xu, C., Song, J., Lee, C.W., Liao, M., Farese Jr., R.V., Walther, T.C.(2023) Nat Commun 14: 3100-3100
- PubMed: 37248213
- DOI: https://doi.org/10.1038/s41467-023-38934-3
- Primary Citation of Related Structures:
8ESM, 8ETM - PubMed Abstract:
Inhibitors of triacylglycerol (TG) synthesis have been developed to treat metabolism-related diseases, but we know little about their mechanisms of action. Here, we report cryo-EM structures of the TG-synthesis enzyme acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1), a membrane bound O-acyltransferase (MBOAT), in complex with two different inhibitors, T863 and DGAT1IN1. Each inhibitor binds DGAT1's fatty acyl-CoA substrate binding tunnel that opens to the cytoplasmic side of the ER. T863 blocks access to the tunnel entrance, whereas DGAT1IN1 extends further into the enzyme, with an amide group interacting with more deeply buried catalytic residues. A survey of DGAT1 inhibitors revealed that this amide group may serve as a common pharmacophore for inhibition of MBOATs. The inhibitors were minimally active against the related MBOAT acyl-CoA:cholesterol acyltransferase 1 (ACAT1), yet a single-residue mutation sensitized ACAT1 for inhibition. Collectively, our studies provide a structural foundation for developing DGAT1 and other MBOAT inhibitors.
Organizational Affiliation:
Department of Molecular Metabolism, Harvard T.H. Chan School of Public Health, Boston, MA, USA.