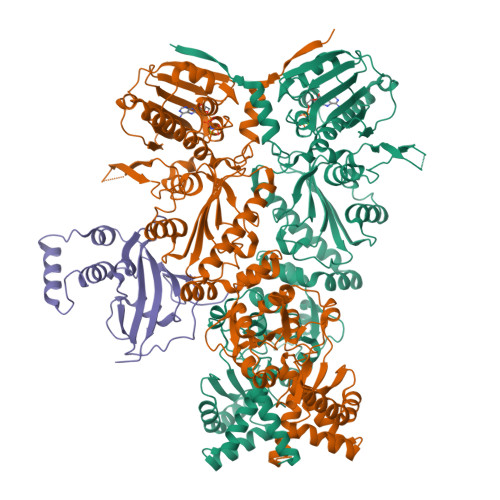
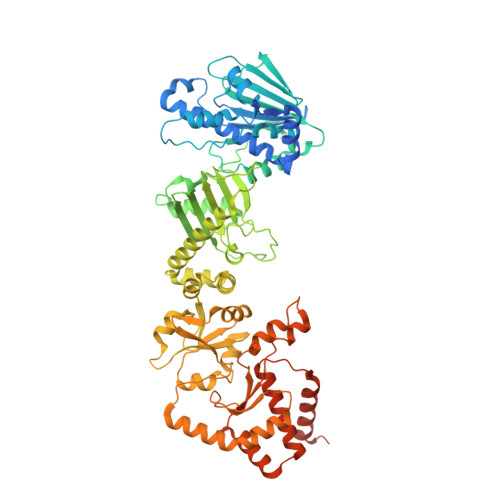
Unique interface and dynamics of the complex of HSP90 with a specialized cochaperone AIPL1.
Srivastava, D., Yadav, R.P., Singh, S., Boyd, K., Artemyev, N.O.(2023) Structure 31: 309
- PubMed: 36657440
- DOI: https://doi.org/10.1016/j.str.2022.12.014
- Primary Citation of Related Structures:
8EOA, 8EOB - PubMed Abstract:
Photoreceptor phosphodiesterase PDE6 is central for visual signal transduction. Maturation of PDE6 depends on a specialized chaperone complex of HSP90 with aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1). Disruption of PDE6 maturation underlies a severe form of retina degeneration. Here, we report a 3.9 Å cryoelectron microscopy (cryo-EM) structure of the complex of HSP90 with AIPL1. This structure reveals a unique interaction of the FK506-binding protein (FKBP)-like domain of AIPL1 with HSP90 at its dimer interface. Unusually, the N terminus AIPL1 inserts into the HSP90 lumen in a manner that was observed previously for HSP90 clients. Deletion of the 7 N-terminal residues of AIPL1 decreased its ability to cochaperone PDE6. Multi-body refinement of the cryo-EM data indicated large swing-like movements of AIPL1-FKBP. Modeling the complex of HSP90 with AIPL1 using crosslinking constraints indicated proximity of the mobile tetratricopeptide repeat (TPR) domain with the C-terminal domain of HSP90. Our study establishes a framework for future structural studies of PDE6 maturation.
Organizational Affiliation:
Department of Molecular Physiology and Biophysics, The University of Iowa Carver College of Medicine, Iowa City, IA 52242, USA.