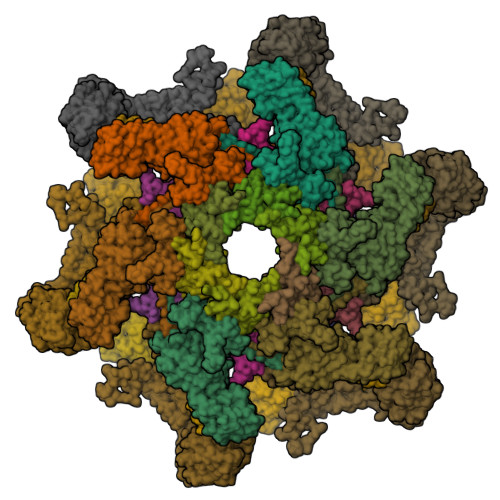
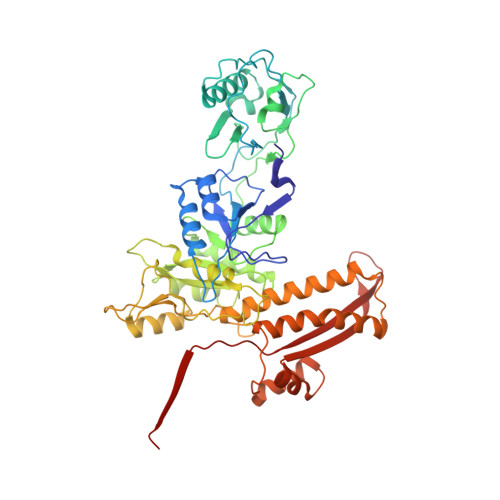
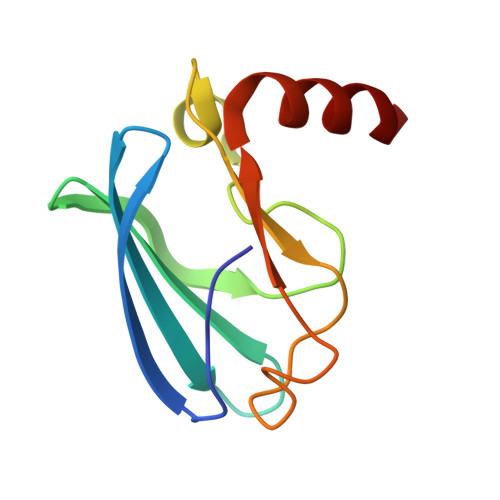
High-resolution cryo-EM structure of the Pseudomonas bacteriophage E217.
Li, F., Hou, C.D., Lokareddy, R.K., Yang, R., Forti, F., Briani, F., Cingolani, G.(2023) Nat Commun 14: 4052-4052
- PubMed: 37422479
- DOI: https://doi.org/10.1038/s41467-023-39756-z
- Primary Citation of Related Structures:
8ENV, 8EON, 8FRS, 8FUV, 8FVG, 8FVH - PubMed Abstract:
E217 is a Pseudomonas phage used in an experimental cocktail to eradicate cystic fibrosis-associated Pseudomonas aeruginosa. Here, we describe the structure of the whole E217 virion before and after DNA ejection at 3.1 Å and 4.5 Å resolution, respectively, determined using cryogenic electron microscopy (cryo-EM). We identify and build de novo structures for 19 unique E217 gene products, resolve the tail genome-ejection machine in both extended and contracted states, and decipher the complete architecture of the baseplate formed by 66 polypeptide chains. We also determine that E217 recognizes the host O-antigen as a receptor, and we resolve the N-terminal portion of the O-antigen-binding tail fiber. We propose that E217 design principles presented in this paper are conserved across PB1-like Myoviridae phages of the Pbunavirus genus that encode a ~1.4 MDa baseplate, dramatically smaller than the coliphage T4.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Thomas Jefferson University, 1020 Locust Street, Philadelphia, PA, 19107, USA.