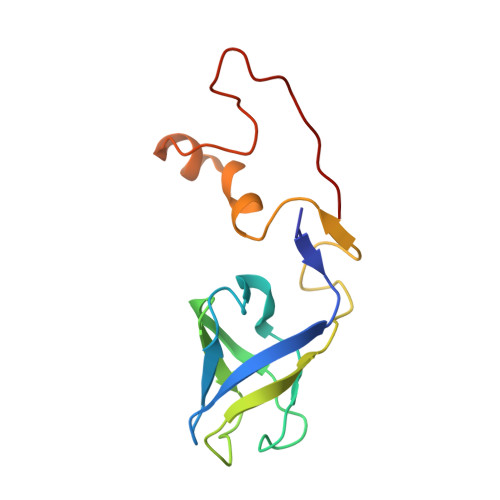
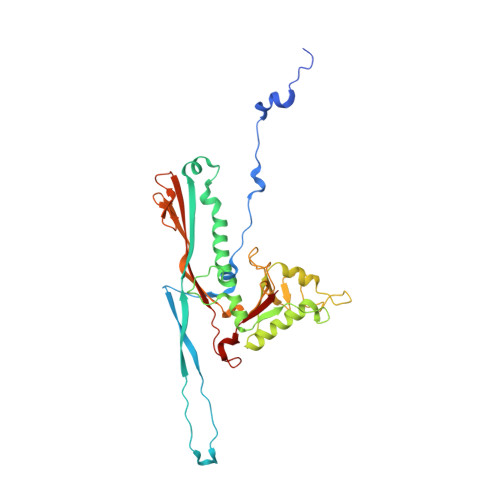
A structural dendrogram of the actinobacteriophage major capsid proteins provides important structural insights into the evolution of capsid stability.
Podgorski, J.M., Freeman, K., Gosselin, S., Huet, A., Conway, J.F., Bird, M., Grecco, J., Patel, S., Jacobs-Sera, D., Hatfull, G., Gogarten, J.P., Ravantti, J., White, S.J.(2023) Structure 31: 282
- PubMed: 36649709
- DOI: https://doi.org/10.1016/j.str.2022.12.012
- Primary Citation of Related Structures:
8E16, 8EB4, 8EC2, 8EC8, 8ECI, 8ECJ, 8ECK, 8ECN, 8ECO, 8EDU - PubMed Abstract:
Many double-stranded DNA viruses, including tailed bacteriophages (phages) and herpesviruses, use the HK97-fold in their major capsid protein to make the capsomers of the icosahedral viral capsid. After the genome packaging at near-crystalline densities, the capsid is subjected to a major expansion and stabilization step that allows it to withstand environmental stresses and internal high pressure. Several different mechanisms for stabilizing the capsid have been structurally characterized, but how these mechanisms have evolved is still not understood. Using cryo-EM structure determination of 10 capsids, structural comparisons, phylogenetic analyses, and Alphafold predictions, we have constructed a detailed structural dendrogram describing the evolution of capsid structural stability within the actinobacteriophages. We show that the actinobacteriophage major capsid proteins can be classified into 15 groups based upon their HK97-fold.
Organizational Affiliation:
Biology/Physics Building, Department of Molecular and Cell Biology, University of Connecticut, 91 North Eagleville Road, Unit-3125, Storrs, CT 06269-3125, USA.