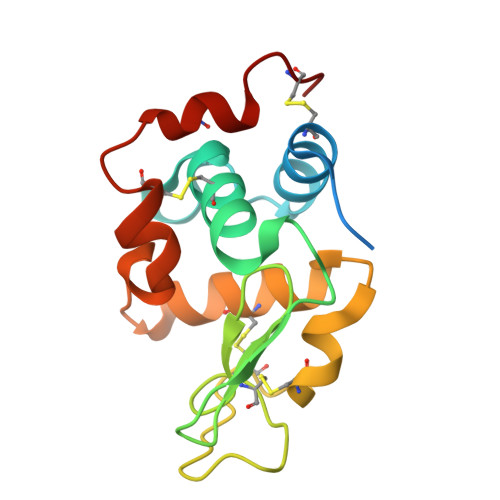
Electron-counting MicroED data with the K2 and K3 direct electron detectors.
Clabbers, M.T.B., Martynowycz, M.W., Hattne, J., Nannenga, B.L., Gonen, T.(2022) J Struct Biol 214: 107886-107886
- PubMed: 36044956
- DOI: https://doi.org/10.1016/j.jsb.2022.107886
- Primary Citation of Related Structures:
8E52, 8E53, 8E54 - PubMed Abstract:
Microcrystal electron diffraction (MicroED) uses electron cryo-microscopy (cryo-EM) to collect diffraction data from small crystals during continuous rotation of the sample. As a result of advances in hardware as well as methods development, the data quality has continuously improved over the past decade, to the point where even macromolecular structures can be determined ab initio. Detectors suitable for electron diffraction should ideally have fast readout to record data in movie mode, and high sensitivity at low exposure rates to accurately report the intensities. Direct electron detectors are commonly used in cryo-EM imaging for their sensitivity and speed, but despite their availability are generally not used in diffraction. Primary concerns with diffraction experiments are the dynamic range and coincidence loss, which will corrupt the measurement if the flux exceeds the count rate of the detector. Here, we describe instrument setup and low-exposure MicroED data collection in electron-counting mode using K2 and K3 direct electron detectors and show that the integrated intensities can be effectively used to solve structures of two macromolecules between 1.2 Å and 2.8 Å resolution. Even though a beam stop was not used with the K3 studies we did not observe damage to the camera. As these cameras are already available in many cryo-EM facilities, this provides opportunities for users who do not have access to dedicated facilities for MicroED.
Organizational Affiliation:
Department of Biological Chemistry, University of California, Los Angeles CA 90095, United States; Howard Hughes Medical Institute, University of California, Los Angeles CA 90095, United States.