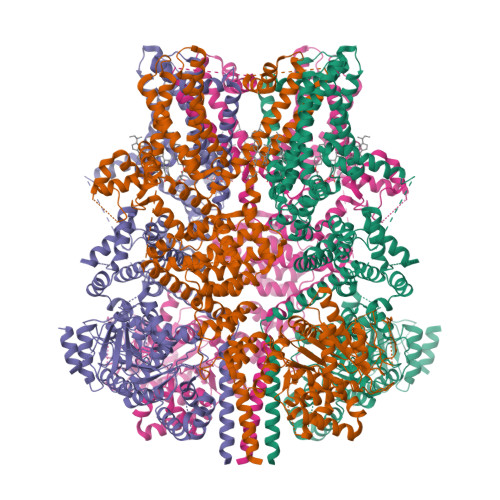
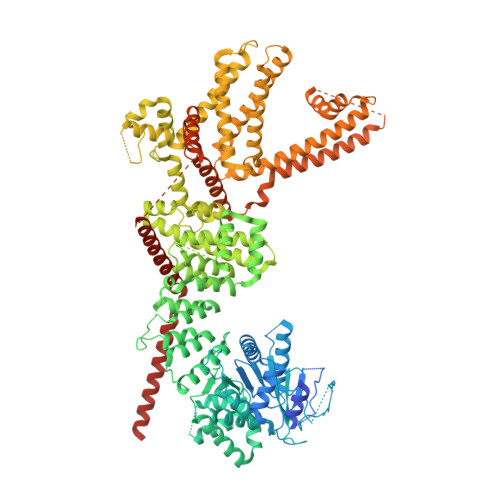
Activation mechanism of the mouse cold-sensing TRPM8 channel by cooling agonist and PIP 2.
Yin, Y., Zhang, F., Feng, S., Butay, K.J., Borgnia, M.J., Im, W., Lee, S.Y.(2022) Science 378: eadd1268-eadd1268
- PubMed: 36227998
- DOI: https://doi.org/10.1126/science.add1268
- Primary Citation of Related Structures:
8E4L, 8E4M, 8E4N, 8E4O, 8E4P, 8E4Q - PubMed Abstract:
The transient receptor potential melastatin 8 (TRPM8) channel is the primary molecular transducer responsible for the cool sensation elicited by menthol and cold in mammals. TRPM8 activation is controlled by cooling compounds together with the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP 2 ). Our knowledge of cold sensation and the therapeutic potential of TRPM8 for neuroinflammatory diseases and pain will be enhanced by understanding the structural basis of cooling agonist- and PIP 2 -dependent TRPM8 activation. We present cryo-electron microscopy structures of mouse TRPM8 in closed, intermediate, and open states along the ligand- and PIP 2 -dependent gating pathway. Our results uncover two discrete agonist sites, state-dependent rearrangements in the gate positions, and a disordered-to-ordered transition of the gate-forming S6-elucidating the molecular basis of chemically induced cool sensation in mammals.
Organizational Affiliation:
Department of Biochemistry, Duke University School of Medicine, Durham, NC 27710, USA.