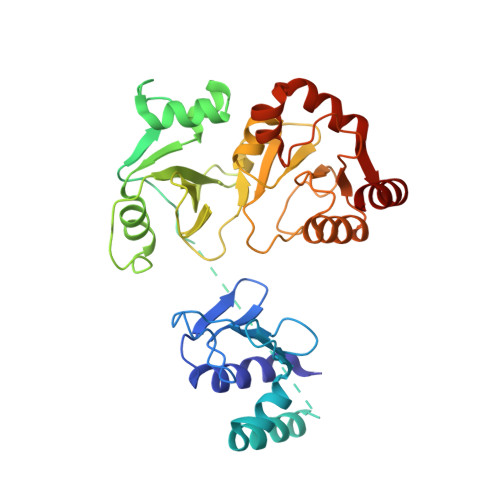
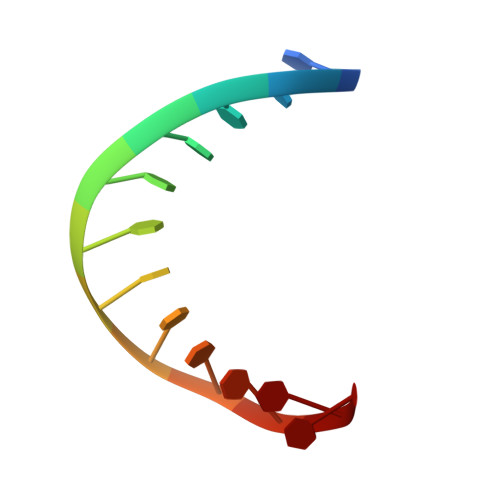
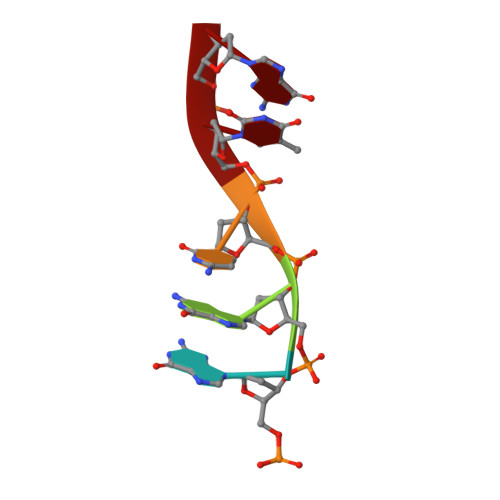
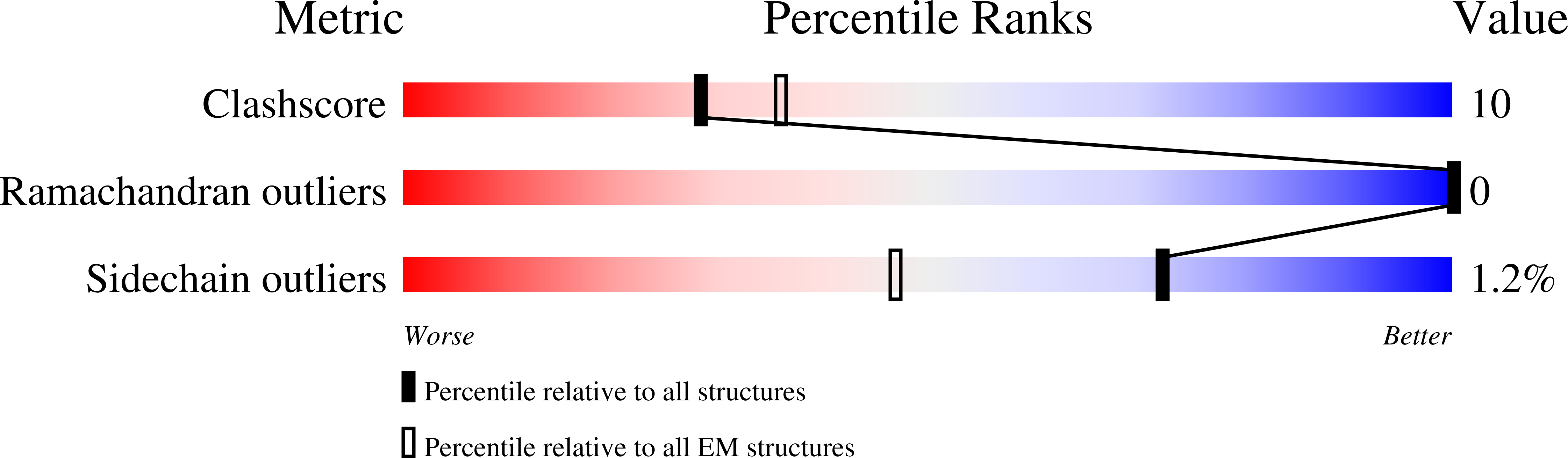Structural basis of the T4 bacteriophage primosome assembly and primer synthesis.
Feng, X., Spiering, M.M., de Luna Almeida Santos, R., Benkovic, S.J., Li, H.(2023) Nat Commun 14: 4396-4396
- PubMed: 37474605
- DOI: https://doi.org/10.1038/s41467-023-40106-2
- Primary Citation of Related Structures:
8DTP, 8DUE, 8DUO, 8DVF, 8DVI, 8DW6, 8DWJ, 8G0Z, 8GAO - PubMed Abstract:
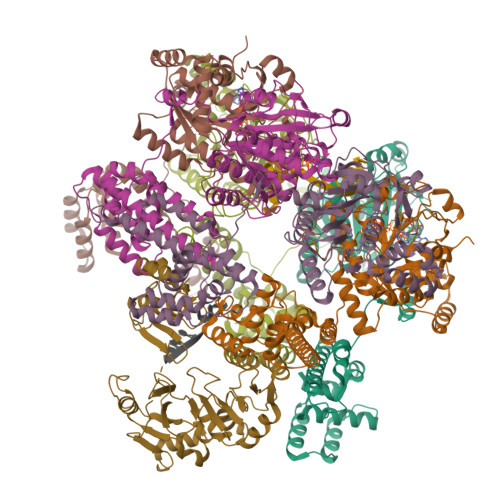
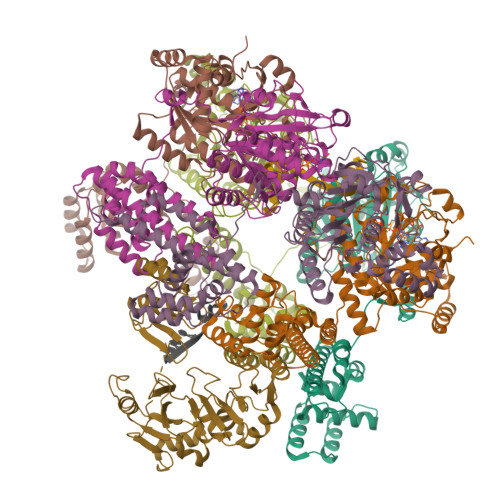
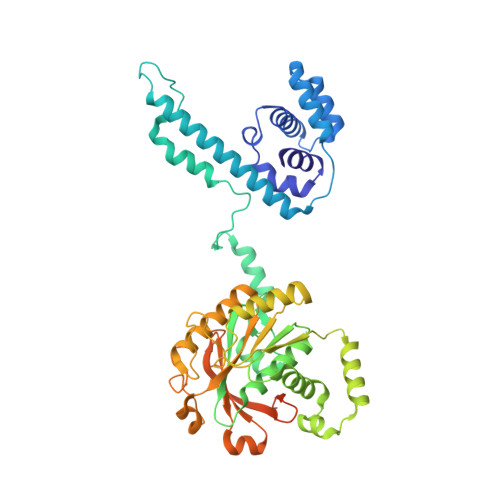
The T4 bacteriophage gp41 helicase and gp61 primase assemble into a primosome to couple DNA unwinding with RNA primer synthesis for DNA replication. How the primosome is assembled and how the primer length is defined are unclear. Here we report a series of cryo-EM structures of T4 primosome assembly intermediates. We show that gp41 alone is an open spiral, and ssDNA binding triggers a large-scale scissor-like conformational change that drives the ring closure and activates the helicase. Helicase activation exposes a cryptic hydrophobic surface to recruit the gp61 primase. The primase binds the helicase in a bipartite mode in which the N-terminal Zn-binding domain and the C-terminal RNA polymerase domain each contain a helicase-interacting motif that bind to separate gp41 N-terminal hairpin dimers, leading to the assembly of one primase on the helicase hexamer. Our study reveals the T4 primosome assembly process and sheds light on the RNA primer synthesis mechanism.
Organizational Affiliation:
Department of Structural Biology, Van Andel Institute, Grand Rapids, MI, USA.