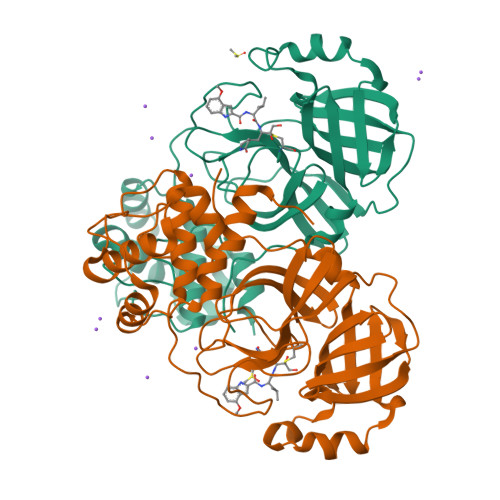
Contributions of Hyperactive Mutations in M pro from SARS-CoV-2 to Drug Resistance.
Flynn, J.M., Zvornicanin, S.N., Tsepal, T., Shaqra, A.M., Kurt Yilmaz, N., Jia, W., Moquin, S., Dovala, D., Schiffer, C.A., Bolon, D.N.A.(2024) ACS Infect Dis 10: 1174-1184
- PubMed: 38472113
- DOI: https://doi.org/10.1021/acsinfecdis.3c00560
- Primary Citation of Related Structures:
8DT9, 8E4W, 8E5C - PubMed Abstract:
The appearance and spread of mutations that cause drug resistance in rapidly evolving diseases, including infections by the SARS-CoV-2 virus, are major concerns for human health. Many drugs target enzymes, and resistance-conferring mutations impact inhibitor binding or enzyme activity. Nirmatrelvir, the most widely used inhibitor currently used to treat SARS-CoV-2 infections, targets the main protease (M pro ) preventing it from processing the viral polyprotein into active subunits. Our previous work systematically analyzed resistance mutations in M pro that reduce binding to inhibitors; here, we investigate mutations that affect enzyme function. Hyperactive mutations that increase M pro activity can contribute to drug resistance but have not been thoroughly studied. To explore how hyperactive mutations contribute to resistance, we comprehensively assessed how all possible individual mutations in M pro affect enzyme function using a mutational scanning approach with a fluorescence resonance energy transfer (FRET)-based yeast readout. We identified hundreds of mutations that significantly increased the M pro activity. Hyperactive mutations occurred both proximal and distal to the active site, consistent with protein stability and/or dynamics impacting activity. Hyperactive mutations were observed 3 times more than mutations which reduced apparent binding to nirmatrelvir in recent studies of laboratory-grown viruses selected for drug resistance. Hyperactive mutations were also about three times more prevalent than nirmatrelvir binding mutations in sequenced isolates from circulating SARS-CoV-2. Our findings indicate that hyperactive mutations are likely to contribute to the natural evolution of drug resistance in M pro and provide a comprehensive list for future surveillance efforts.
Organizational Affiliation:
Department of Biochemistry and Molecular Biotechnology, University of Massachusetts Chan Medical School, Worcester, Massachusetts 01605, United States.