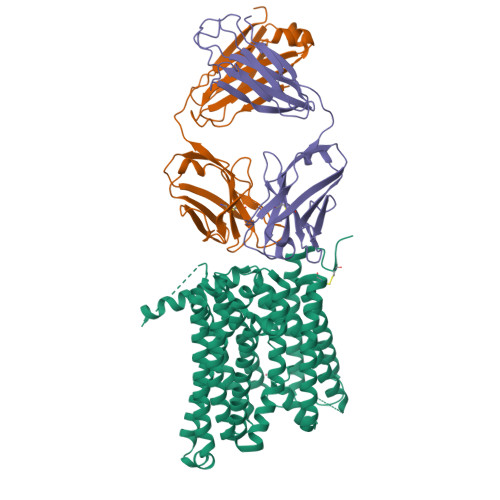
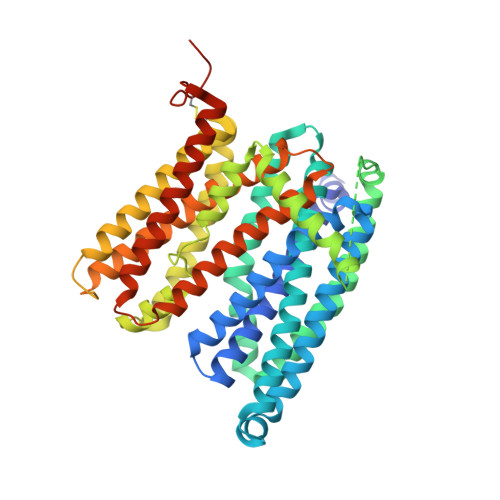
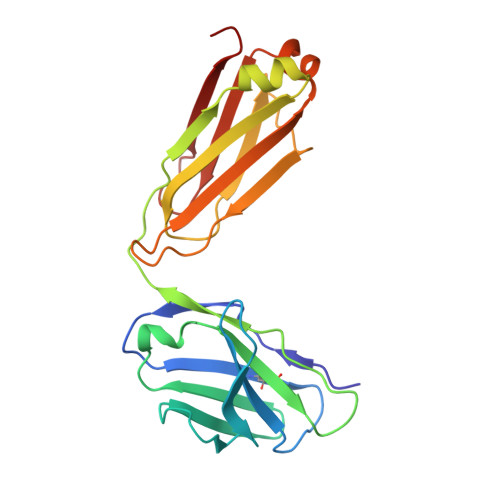
Structural basis of ferroportin inhibition by minihepcidin PR73.
Wilbon, A.S., Shen, J., Ruchala, P., Zhou, M., Pan, Y.(2023) PLoS Biol 21: e3001936-e3001936
- PubMed: 36649314
- DOI: https://doi.org/10.1371/journal.pbio.3001936
- Primary Citation of Related Structures:
8DL7, 8DL8 - PubMed Abstract:
Ferroportin (Fpn) is the only known iron exporter in humans and is essential for maintaining iron homeostasis. Fpn activity is suppressed by hepcidin, an endogenous peptide hormone, which inhibits iron export and promotes endocytosis of Fpn. Hepcidin deficiency leads to hemochromatosis and iron-loading anemia. Previous studies have shown that small peptides that mimic the first few residues of hepcidin, i.e., minihepcidins, are more potent than hepcidin. However, the mechanism of enhanced inhibition by minihepcidins remains unclear. Here, we report the structure of human ferroportin in complex with a minihepcidin, PR73 that mimics the first 9 residues of hepcidin, at 2.7 Å overall resolution. The structure reveals novel interactions that were not present between Fpn and hepcidin. We validate PR73-Fpn interactions through binding and transport assays. These results provide insights into how minihepcidins increase inhibition potency and will guide future development of Fpn inhibitors.
Organizational Affiliation:
Verna and Marrs McLean Department of Biochemistry and Molecular Biology, Baylor College of Medicine, Houston, Texas, United States of America.