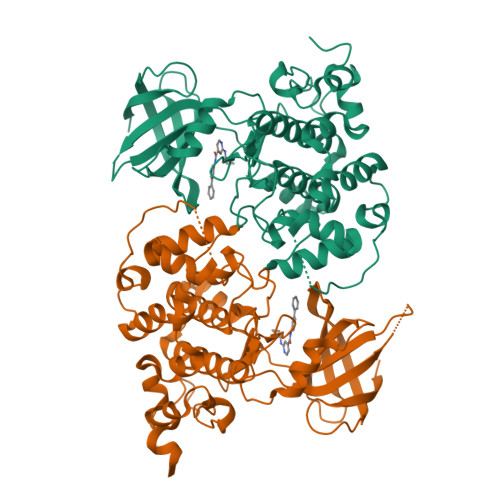
Structure-activity relationship (SAR) studies on substituted N-(pyridin-3-yl)-2-amino-isonicotinamides as highly potent and selective glycogen synthase kinase-3 (GSK-3) inhibitors.
Luo, G., Chen, L., Jacutin-Porte, S., Han, Y., Burton, C.R., Xiao, H., Krause, C.M., Cao, Y., Liu, N., Kish, K., Lewis, H.A., Macor, J.E., Dubowchik, G.M.(2023) Bioorg Med Chem Lett 81: 129143-129143
- PubMed: 36669575
- DOI: https://doi.org/10.1016/j.bmcl.2023.129143
- Primary Citation of Related Structures:
8DJD - PubMed Abstract:
In our continuing efforts to explore structure-activity relationships around the novel class of potent, isonicotinamide-based GSK3 inhibitors described in our previous report, we extensively explored structural variations around both 4/5-pyridine substitutions and the amide group. Some analogs were found to have greatly improved pTau lowering potency while retaining high kinase selectivity. In contrast to previous active compounds 1a-c, a close analog 3h did not show in vivo efficacy in a triple-transgenic mouse Alzheimer's disease model. In general, these 2‑pyridinyl amide derivatives were prone to amidase mediated hydrolysis in mouse plasma.
Organizational Affiliation:
Bristol-Myers Squibb, Wallingford, CT 06492, United States; Bristol Myers Squibb, Lawrenceville, NJ 08543, United States. Electronic address: guanglin.luo@bms.com.