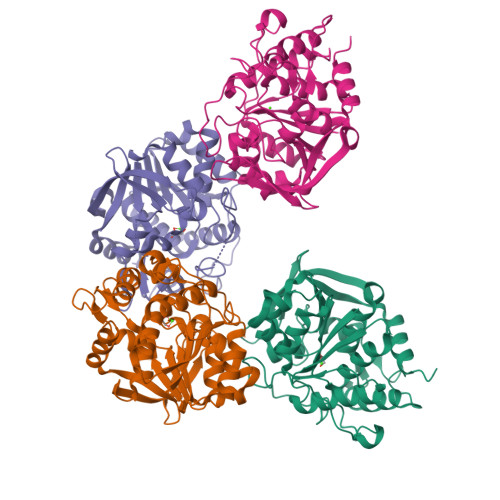
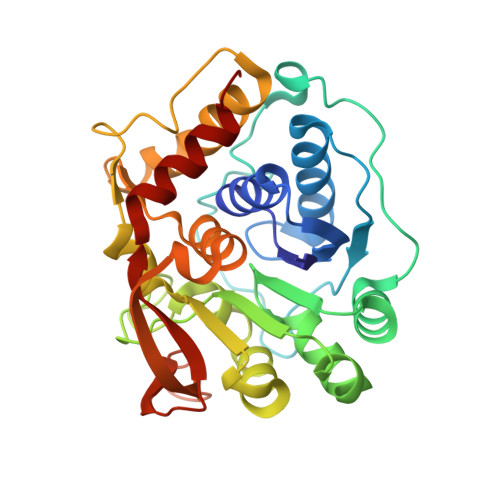
Structure-Guided Insight into the Specificity and Mechanism of a Parasitic Nucleoside Hydrolase.
Muellers, S.N., Nyitray, M.M., Reynarowych, N., Saljanin, E., Benzie, A.L., Schoenfeld, A.R., Stockman, B.J., Allen, K.N.(2022) Biochemistry 61: 1853-1861
- PubMed: 35994320
- DOI: https://doi.org/10.1021/acs.biochem.2c00361
- Primary Citation of Related Structures:
8DB6, 8DB7, 8DB8, 8DB9 - PubMed Abstract:
Trichomonas vaginalis is the causative parasitic protozoan of the disease trichomoniasis, the most prevalent, nonviral sexually transmitted disease in the world. T. vaginalis is a parasite that scavenges nucleosides from the host organism via catalysis by nucleoside hydrolase (NH) enzymes to yield purine and pyrimidine bases. One of the four NH enzymes identified within the genome of T. vaginalis displays unique specificity toward purine nucleosides, adenosine and guanosine, but not inosine, and atypically shares greater sequence similarity to the pyrimidine hydrolases. Bioinformatic analysis of this enzyme, adenosine/guanosine-preferring nucleoside ribohydrolase (AGNH), was incapable of identifying the residues responsible for this uncommon specificity, highlighting the need for structural information. Here, we report the X-ray crystal structures of holo , unliganded AGNH and three additional structures of the enzyme bound to fragment and small-molecule inhibitors. Taken together, these structures facilitated the identification of residue Asp231, which engages in substrate interactions in the absence of those residues that typically support the canonical purine-specific tryptophan-stacking specificity motif. An altered substrate-binding pose is mirrored by repositioning within the protein scaffold of the His80 general acid/base catalyst. The newly defined structure-determined sequence markers allowed the assignment of additional NH orthologs, which are proposed to exhibit the same specificity for adenosine and guanosine alone and further delineate specificity classes for these enzymes.
Organizational Affiliation:
Department of Chemistry, Boston University, Boston, Massachusetts 02215, United States.