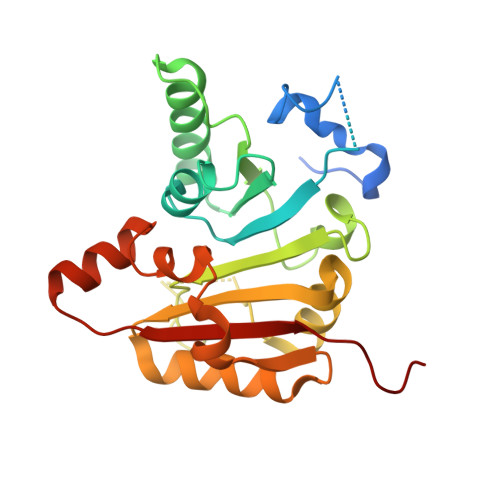
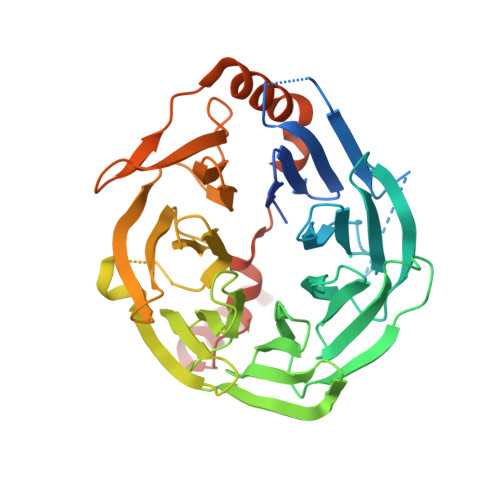
Structures and mechanisms of tRNA methylation by METTL1-WDR4.
Ruiz-Arroyo, V.M., Raj, R., Babu, K., Onolbaatar, O., Roberts, P.H., Nam, Y.(2023) Nature 613: 383-390
- PubMed: 36599982
- DOI: https://doi.org/10.1038/s41586-022-05565-5
- Primary Citation of Related Structures:
8D58, 8D59, 8D5B, 8D9K, 8D9L, 8EG0 - PubMed Abstract:
Specific, regulated modification of RNAs is important for proper gene expression 1,2 . tRNAs are rich with various chemical modifications that affect their stability and function 3,4 . 7-Methylguanosine (m 7 G) at tRNA position 46 is a conserved modification that modulates steady-state tRNA levels to affect cell growth 5,6 . The METTL1-WDR4 complex generates m 7 G46 in humans, and dysregulation of METTL1-WDR4 has been linked to brain malformation and multiple cancers 7-22 . Here we show how METTL1 and WDR4 cooperate to recognize RNA substrates and catalyse methylation. A crystal structure of METTL1-WDR4 and cryo-electron microscopy structures of METTL1-WDR4-tRNA show that the composite protein surface recognizes the tRNA elbow through shape complementarity. The cryo-electron microscopy structures of METTL1-WDR4-tRNA with S-adenosylmethionine or S-adenosylhomocysteine along with METTL1 crystal structures provide additional insights into the catalytic mechanism by revealing the active site in multiple states. The METTL1 N terminus couples cofactor binding with conformational changes in the tRNA, the catalytic loop and the WDR4 C terminus, acting as the switch to activate m 7 G methylation. Thus, our structural models explain how post-translational modifications of the METTL1 N terminus can regulate methylation. Together, our work elucidates the core and regulatory mechanisms underlying m 7 G modification by METTL1, providing the framework to understand its contribution to biology and disease.
Organizational Affiliation:
Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX, USA.