Optimization of a Class of Dihydrobenzofurane Analogs toward Orally Efficacious YAP-TEAD Protein-Protein Interaction Inhibitors.
Sellner, H., Chapeau, E., Furet, P., Voegtle, M., Salem, B., Le Douget, M., Bordas, V., Groell, J.M., Le Goff, A.L., Rouzet, C., Wietlisbach, T., Zimmermann, T., McKenna, J., Brocklehurst, C.E., Chene, P., Wartmann, M., Scheufler, C., Kallen, J., Williams, G., Harlfinger, S., Traebert, M., Dumotier, B.M., Schmelzle, T., Soldermann, N.(2023) ChemMedChem 18: e202300051-e202300051
- PubMed: 36988034
- DOI: https://doi.org/10.1002/cmdc.202300051
- Primary Citation of Related Structures:
8CAA - PubMed Abstract:
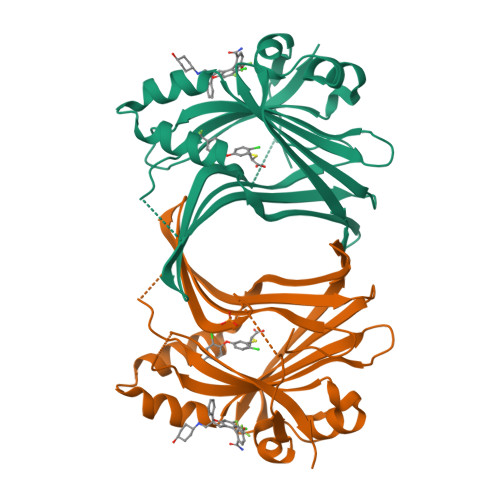
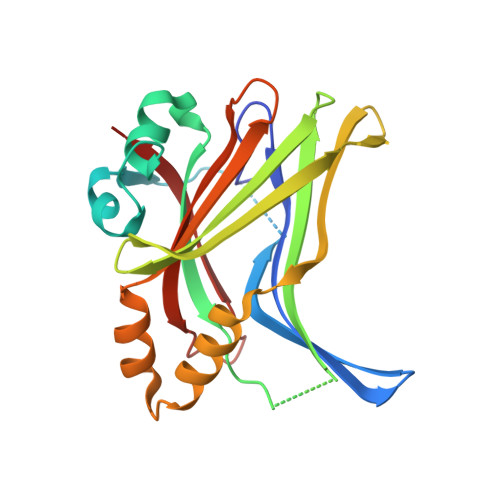
The inhibition of the YAP-TEAD protein-protein interaction constitutes a promising therapeutic approach for the treatment of cancers linked to the dysregulation of the Hippo signaling pathway. The identification of a class of small molecules which potently inhibit the YAP-TEAD interaction by binding tightly to the Ω-loop pocket of TEAD has previously been communicated. This report details the further multi-parameter optimization of this class of compounds resulting in advanced analogs combining nanomolar cellular potency with a balanced ADME and off-target profile, and efficacy of these compounds in tumor bearing mice is demonstrated for the first time.
Organizational Affiliation:
Global Discovery Chemistry, Novartis Institutes for BioMedical Research, Basel, 4002, Switzerland.