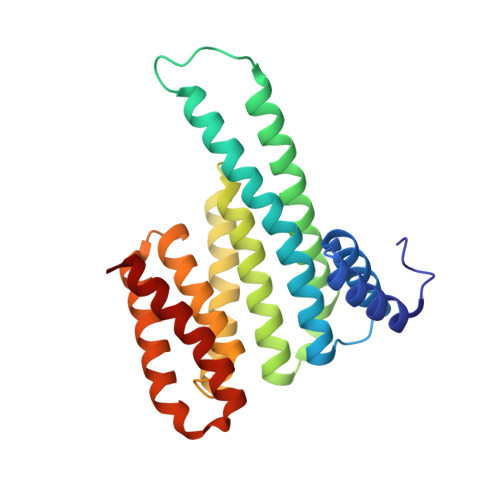
Molecular basis and dual ligand regulation of tetrameric estrogen receptor alpha /14-3-3 zeta protein complex.
Somsen, B.A., Sijbesma, E., Leysen, S., Honzejkova, K., Visser, E.J., Cossar, P.J., Obsil, T., Brunsveld, L., Ottmann, C.(2023) J Biol Chem 299: 104855-104855
- PubMed: 37224961
- DOI: https://doi.org/10.1016/j.jbc.2023.104855
- Primary Citation of Related Structures:
8C3Z, 8C40, 8C42, 8C43 - PubMed Abstract:
Therapeutic strategies targeting nuclear receptors (NRs) beyond their endogenous ligand binding pocket have gained significant scientific interest driven by a need to circumvent problems associated with drug resistance and pharmacological profile. The hub protein 14-3-3 is an endogenous regulator of various NRs, providing a novel entry point for small molecule modulation of NR activity. Exemplified, 14-3-3 binding to the C-terminal F-domain of the estrogen receptor alpha (ERα), and small molecule stabilization of the ERα/14-3-3ζ protein complex by the natural product Fusicoccin A (FC-A), was demonstrated to downregulate ERα-mediated breast cancer proliferation. This presents a novel drug discovery approach to target ERα; however, structural and mechanistic insights into ERα/14-3-3 complex formation are lacking. Here, we provide an in-depth molecular understanding of the ERα/14-3-3ζ complex by isolating 14-3-3ζ in complex with an ERα protein construct comprising its ligand-binding domain (LBD) and phosphorylated F-domain. Bacterial co-expression and co-purification of the ERα/14-3-3ζ complex, followed by extensive biophysical and structural characterization, revealed a tetrameric complex between the ERα homodimer and the 14-3-3ζ homodimer. 14-3-3ζ binding to ERα, and ERα/14-3-3ζ complex stabilization by FC-A, appeared to be orthogonal to ERα endogenous agonist (E2) binding, E2-induced conformational changes, and cofactor recruitment. Similarly, the ERα antagonist 4-hydroxytamoxifen inhibited cofactor recruitment to the ERα LBD while ERα was bound to 14-3-3ζ. Furthermore, stabilization of the ERα/14-3-3ζ protein complex by FC-A was not influenced by the disease-associated and 4-hydroxytamoxifen resistant ERα-Y537S mutant. Together, these molecular and mechanistic insights provide direction for targeting ERα via the ERα/14-3-3 complex as an alternative drug discovery approach.
Organizational Affiliation:
Department of Biomedical Engineering and Institute for Complex Molecular Systems, Laboratory of Chemical Biology, Eindhoven University of Technology, Eindhoven, The Netherlands.