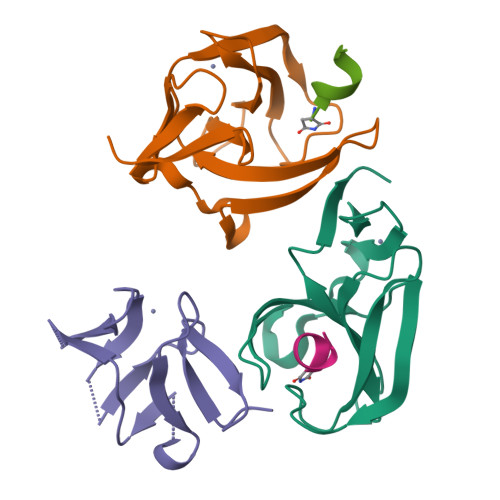
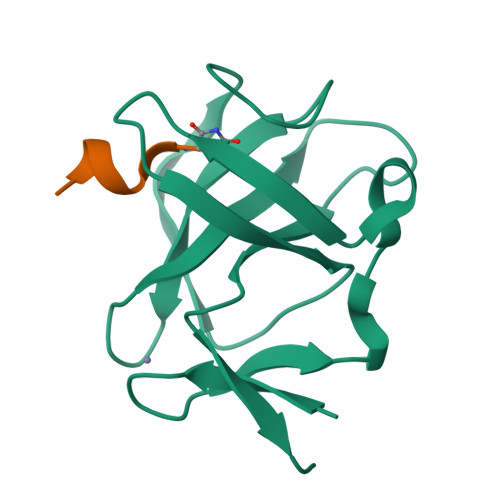
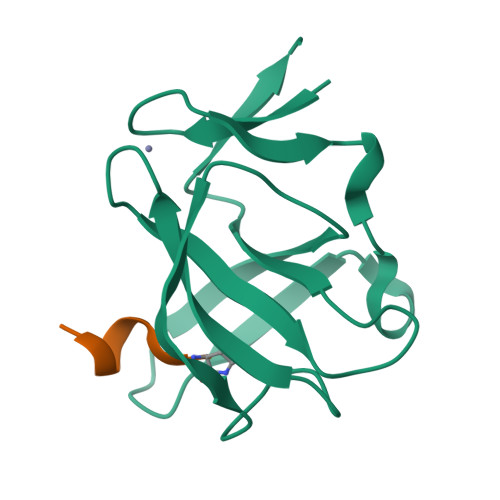
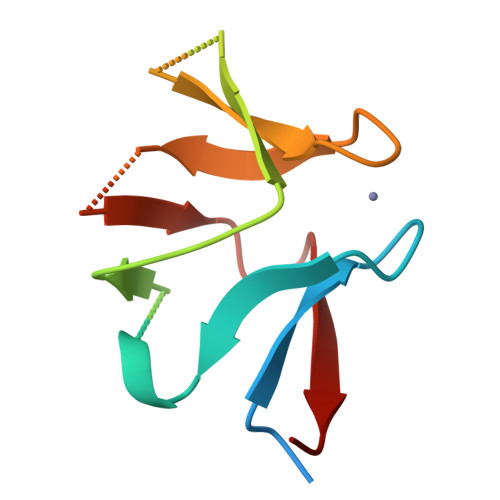
Cereblon neo-substrate binding mimics the recognition of the cyclic imide degron.
Heim, C., Spring, A.K., Kirchgassner, S., Schwarzer, D., Hartmann, M.D.(2023) Biochem Biophys Res Commun 646: 30-35
- PubMed: 36701892
- DOI: https://doi.org/10.1016/j.bbrc.2023.01.051
- Primary Citation of Related Structures:
8C3H - PubMed Abstract:
In targeted protein degradation, immunomodulatory drugs (IMiDs) or cereblon (CRBN) E3 ligase modulatory drugs (CELMoDs) recruit neo-substrate proteins to the E3 ubiquitin ligase receptor CRBN for ubiquitination and subsequent proteasomal degradation. While the structural basis of this mechanism is generally understood, we have only recently described the recognition mode of the natural CRBN degron. In this communication, we reveal that the IMiD- or CELMoD-mediated binding of neo-substrates closely mimics the recognition of natural degrons. In crystal structures, we identify a conserved binding mode for natural degron peptides with an elaborate hydrogen bonding network involving the backbone of each of the six C-terminal degron residues, without the involvement of side chains. In a structural comparison, we show that neo-substrates recruited by IMiDs or CELMoDs emulate every single hydrogen bond of this network and thereby explain the origins of the largely sequence-independent recognition of neo-substrates. Our results imply that the V388I substitution in CRBN does not impair natural degron recognition and complete the structural basis for the rational design of CRBN effectors.
Organizational Affiliation:
Max Planck Institute for Biology, Tübingen, Germany; Interfaculty Institute of Biochemistry, University of Tübingen, Tübingen, Germany; NanoTemper Technologies GmbH, Munich, Germany.