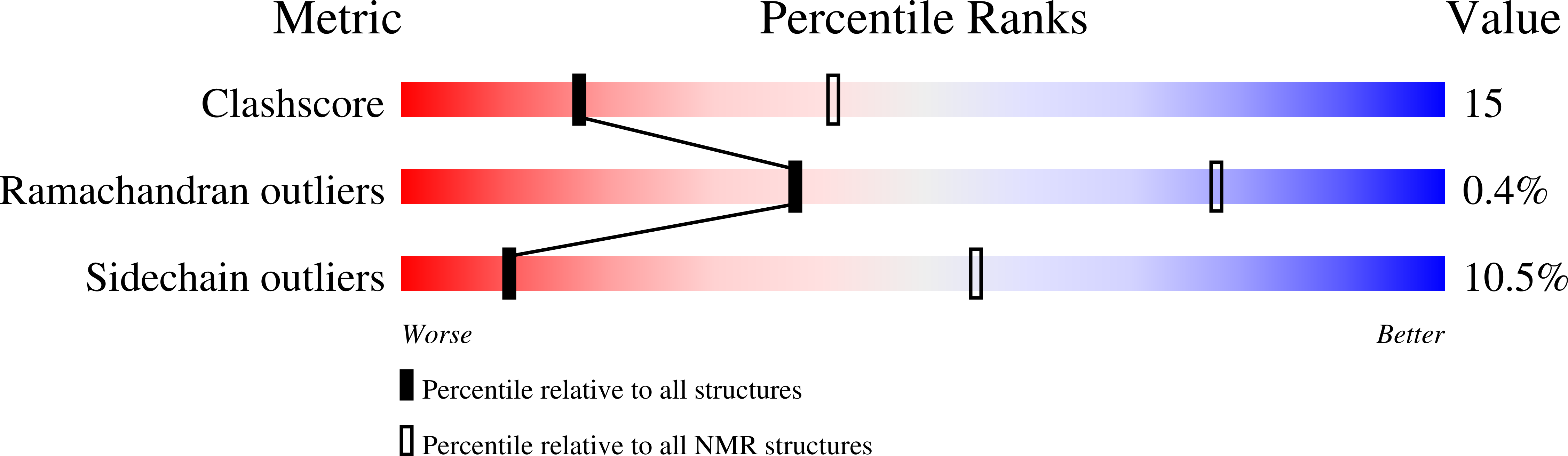Structural basis for the ligand promiscuity of the neofunctionalized, carotenoid-binding fasciclin domain protein AstaP.
Kornilov, F.D., Slonimskiy, Y.B., Lunegova, D.A., Egorkin, N.A., Savitskaya, A.G., Kleymenov, S.Y., Maksimov, E.G., Goncharuk, S.A., Mineev, K.S., Sluchanko, N.N.(2023) Commun Biol 6: 471-471
- PubMed: 37117801
- DOI: https://doi.org/10.1038/s42003-023-04832-z
- Primary Citation of Related Structures:
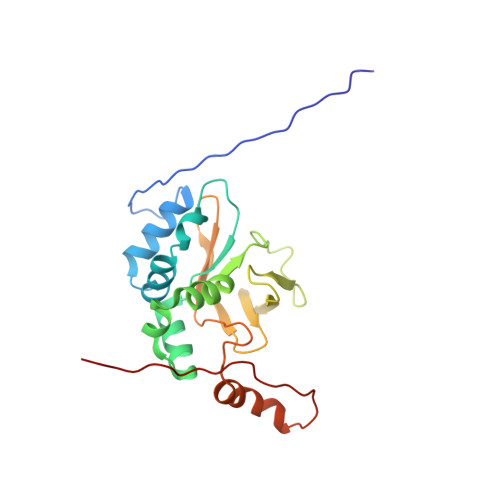
8C18 - PubMed Abstract:
Fasciclins (FAS1) are ancient adhesion protein domains with no common small ligand binding reported. A unique microalgal FAS1-containing astaxanthin (AXT)-binding protein (AstaP) binds a broad repertoire of carotenoids by a largely unknown mechanism. Here, we explain the ligand promiscuity of AstaP-orange1 (AstaPo1) by determining its NMR structure in complex with AXT and validating this structure by SAXS, calorimetry, optical spectroscopy and mutagenesis. α1-α2 helices of the AstaPo1 FAS1 domain embrace the carotenoid polyene like a jaw, forming a hydrophobic tunnel, too short to cap the AXT β-ionone rings and dictate specificity. AXT-contacting AstaPo1 residues exhibit different conservation in AstaPs with the tentative carotenoid-binding function and in FAS1 proteins generally, which supports the idea of AstaP neofunctionalization within green algae. Intriguingly, a cyanobacterial homolog with a similar domain structure cannot bind carotenoids under identical conditions. These structure-activity relationships provide the first step towards the sequence-based prediction of the carotenoid-binding FAS1 members.
Organizational Affiliation:
Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, 117997, Moscow, Russia.