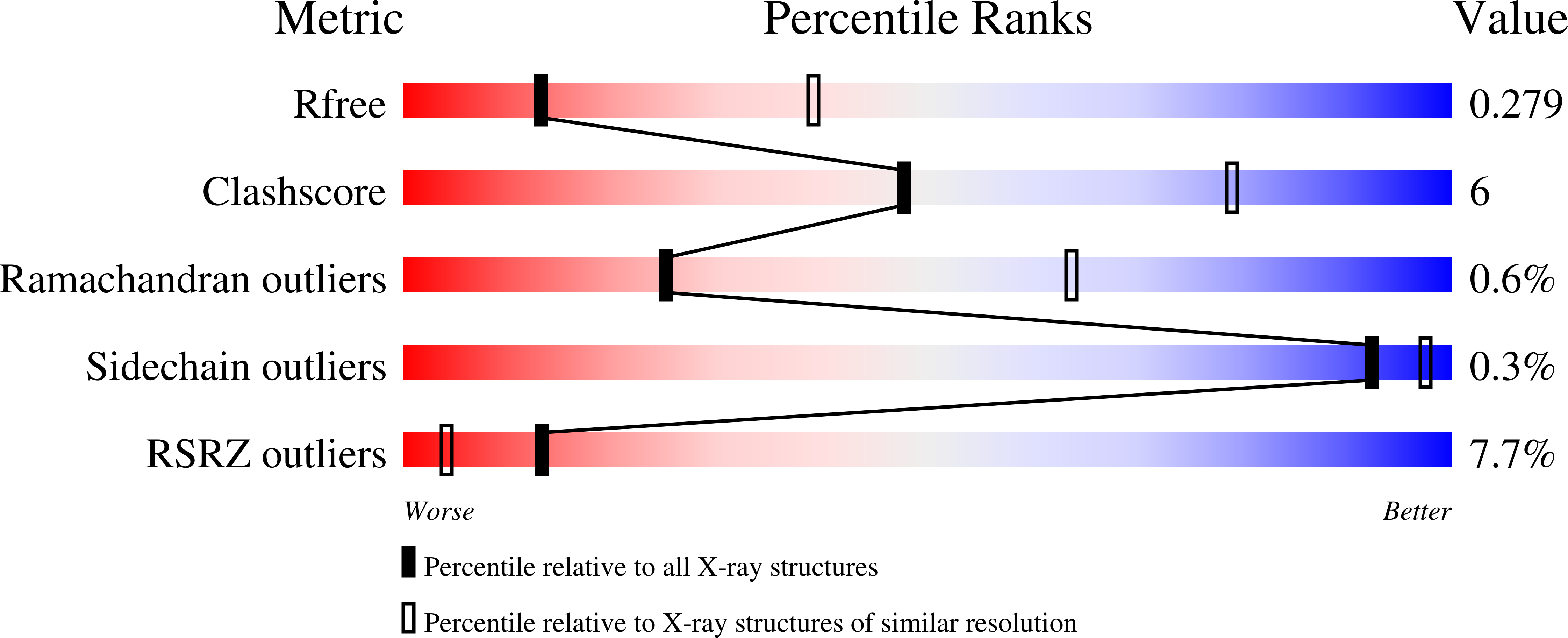
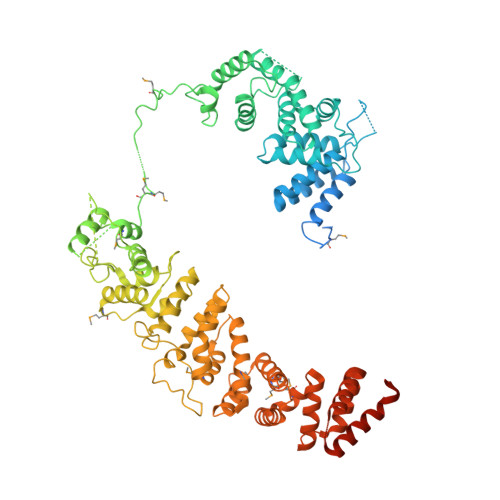
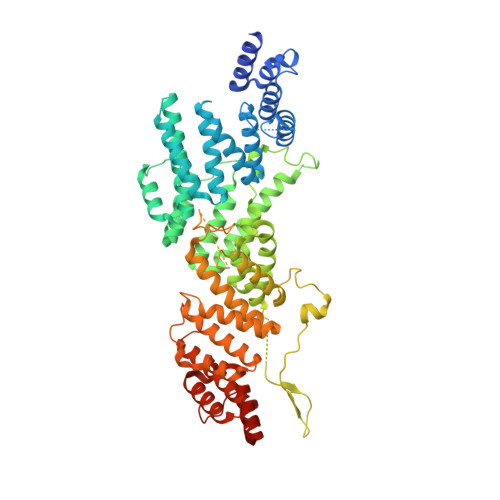
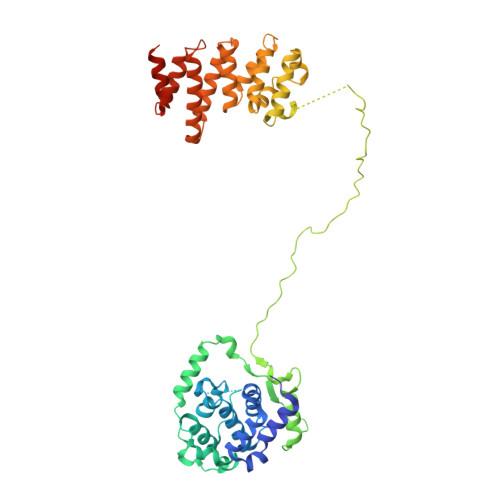
The human CNOT1-CNOT10-CNOT11 complex forms a structural platform for protein-protein interactions.
Mauxion, F., Basquin, J., Ozgur, S., Rame, M., Albrecht, J., Schafer, I., Seraphin, B., Conti, E.(2023) Cell Rep 42: 111902-111902
- PubMed: 36586408
- DOI: https://doi.org/10.1016/j.celrep.2022.111902
- Primary Citation of Related Structures:
8BFH, 8BFI, 8BFJ - PubMed Abstract:
The evolutionary conserved CCR4-NOT complex functions in the cytoplasm as the main mRNA deadenylase in both constitutive mRNA turnover and regulated mRNA decay pathways. The versatility of this complex is underpinned by its modular multi-subunit organization, with distinct structural modules actuating different functions. The structure and function of all modules are known, except for that of the N-terminal module. Using different structural approaches, we obtained high-resolution data revealing the architecture of the human N-terminal module composed of CNOT1, CNOT10, and CNOT11. The structure shows how two helical domains of CNOT1 sandwich CNOT10 and CNOT11, leaving the most conserved domain of CNOT11 protruding into solvent as an antenna. We discovered that GGNBP2, a protein identified as a tumor suppressor and spermatogenic factor, is a conserved interacting partner of the CNOT11 antenna domain. Structural and biochemical analyses thus pinpoint the N-terminal CNOT1-CNOT10-CNOT11 module as a conserved protein-protein interaction platform.
Organizational Affiliation:
Institut de Génétique et de Biologie Moléculaire et cellulaire (IGBMC), Centre National de Recherche scientifique (CNRS) UMR 7104 - Institut National de santé et de Recherche Médicale (Inserm) U964 - Université de Strasbourg, 1 rue Laurent Fries, Illkirch, France. Electronic address: mauxion@igbmc.fr.