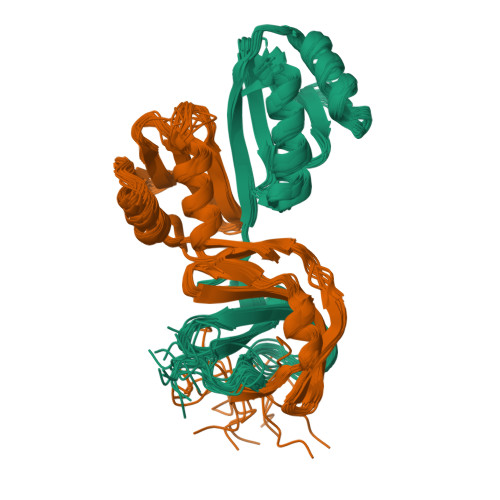
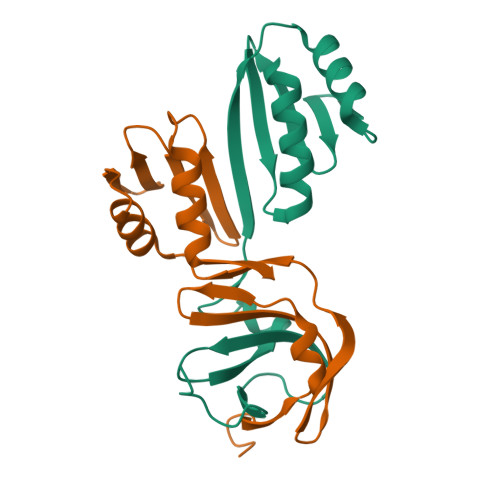
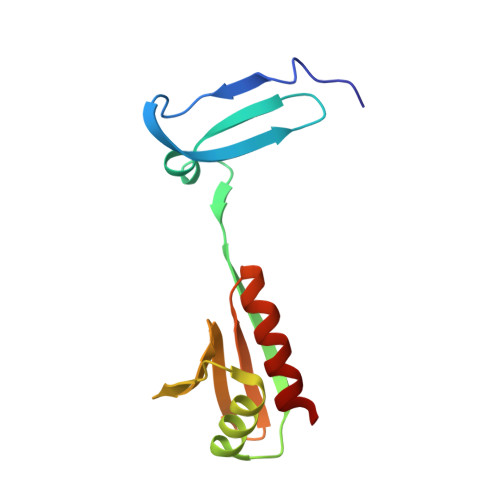
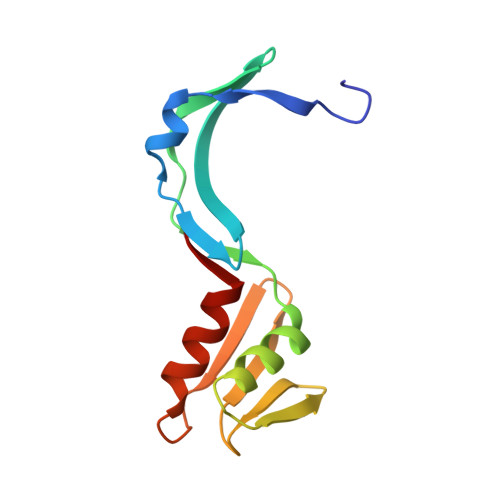
Molecular details of the CPSF73-CPSF100 C-terminal heterodimer and interaction with Symplekin.
Thore, S., Raoelijaona, F., Talenton, V., Fribourg, S., Mackereth, C.D.(2023) Open Biol 13: 230221-230221
- PubMed: 37989222
- DOI: https://doi.org/10.1098/rsob.230221
- Primary Citation of Related Structures:
8B7T, 8BA1 - PubMed Abstract:
Eukaryotic pre-mRNA is processed by a large multiprotein complex to accurately cleave the 3' end, and to catalyse the addition of the poly(A) tail. Within this cleavage and polyadenylation specificity factor (CPSF) machinery, the CPSF73/CPSF3 endonuclease subunit directly contacts both CPSF100/CPSF2 and the scaffold protein Symplekin to form a subcomplex known as the core cleavage complex or mammalian cleavage factor. Here we have taken advantage of a stable CPSF73-CPSF100 minimal heterodimer from Encephalitozoon cuniculi to determine the solution structure formed by the first and second C-terminal domain (CTD1 and CTD2) of both proteins. We find a large number of contacts between both proteins in the complex, and notably in the region between CTD1 and CTD2. A similarity is also observed between CTD2 and the TATA-box binding protein (TBP) domains. Separately, we have determined the structure of the terminal CTD3 domain of CPSF73, which also belongs to the TBP domain family and is connected by a flexible linker to the rest of CPSF73. Biochemical assays demonstrate a key role for the CTD3 of CPSF73 in binding Symplekin, and structural models of the trimeric complex from other species allow for comparative analysis and support an overall conserved architecture.
Organizational Affiliation:
Inserm, CNRS, ARNA Laboratory, Univ. Bordeaux, U1212, UMR 5320, 33000 Bordeaux, France.