Bacterial Dehydrogenases Facilitate Oxidative Inactivation and Bioremediation of Chloramphenicol.
Zhang, L., Toplak, M., Saleem-Batcha, R., Hoing, L., Jakob, R., Jehmlich, N., von Bergen, M., Maier, T., Teufel, R.(2023) Chembiochem 24: e202200632-e202200632
- PubMed: 36353978
- DOI: https://doi.org/10.1002/cbic.202200632
- Primary Citation of Related Structures:
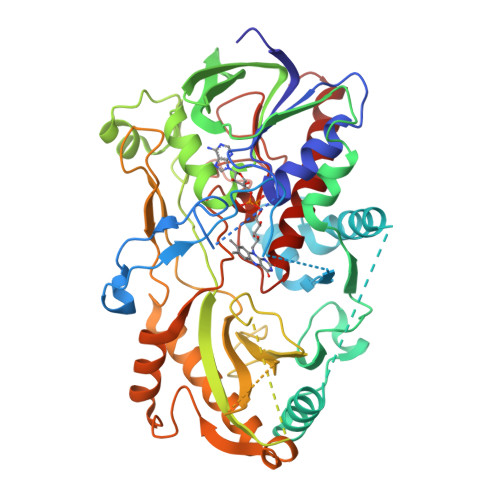
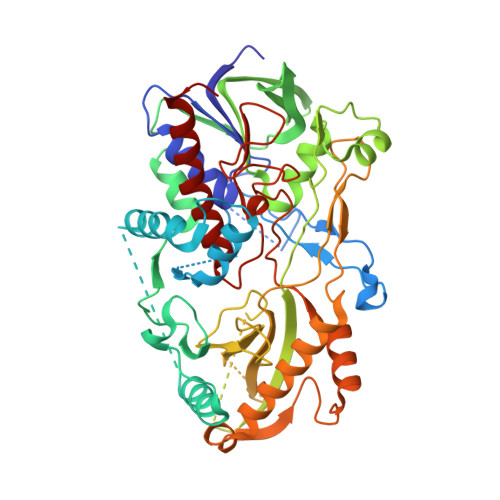
8B7S - PubMed Abstract:
Antimicrobial resistance represents a major threat to human health and knowledge of the underlying mechanisms is therefore vital. Here, we report the discovery and characterization of oxidoreductases that inactivate the broad-spectrum antibiotic chloramphenicol via dual oxidation of the C3-hydroxyl group. Accordingly, chloramphenicol oxidation either depends on standalone glucose-methanol-choline (GMC)-type flavoenzymes, or on additional aldehyde dehydrogenases that boost overall turnover. These enzymes also enable the inactivation of the chloramphenicol analogues thiamphenicol and azidamfenicol, but not of the C3-fluorinated florfenicol. Notably, distinct isofunctional enzymes can be found in Gram-positive (e. g., Streptomyces sp.) and Gram-negative (e. g., Sphingobium sp.) bacteria, which presumably evolved their selectivity for chloramphenicol independently based on phylogenetic analyses. Mechanistic and structural studies provide further insights into the catalytic mechanisms of these biotechnologically interesting enzymes, which, in sum, are both a curse and a blessing by contributing to the spread of antibiotic resistance as well as to the bioremediation of chloramphenicol.
Organizational Affiliation:
Faculty of Biology, University of Freiburg, Schänzlestrasse 1, 79104, Freiburg, Germany.