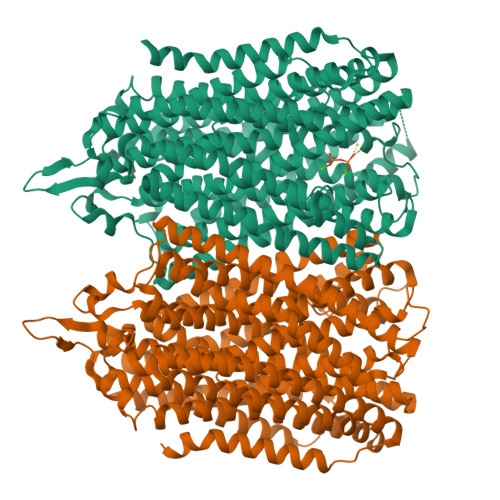
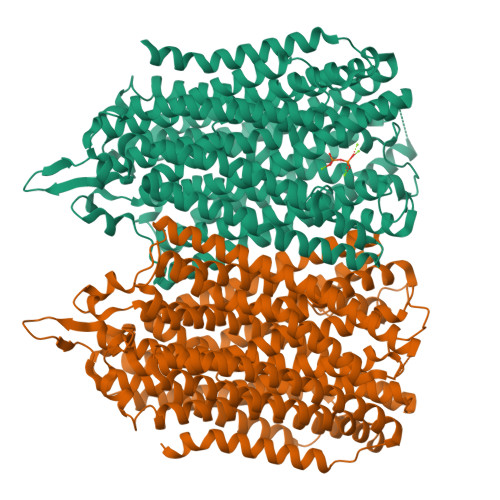
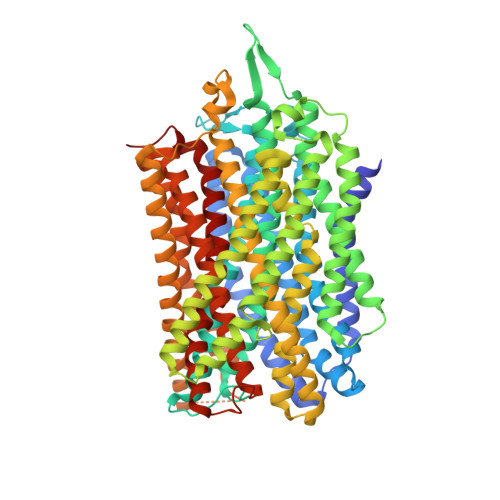
Functional and structural asymmetry suggest a unifying principle for catalysis in membrane-bound pyrophosphatases.
Strauss, J., Wilkinson, C., Vidilaseris, K., de Castro Ribeiro, O.M., Liu, J., Hillier, J., Wichert, M., Malinen, A.M., Gehl, B., Jeuken, L.J., Pearson, A.R., Goldman, A.(2024) EMBO Rep 25: 853-875
- PubMed: 38182815
- DOI: https://doi.org/10.1038/s44319-023-00037-x
- Primary Citation of Related Structures:
8B21, 8B22, 8B23, 8B24, 8B37 - PubMed Abstract:
Membrane-bound pyrophosphatases (M-PPases) are homodimeric primary ion pumps that couple the transport of Na + - and/or H + across membranes to the hydrolysis of pyrophosphate. Their role in the virulence of protist pathogens like Plasmodium falciparum makes them an intriguing target for structural and functional studies. Here, we show the first structure of a K + -independent M-PPase, asymmetric and time-dependent substrate binding in time-resolved structures of a K + -dependent M-PPase and demonstrate pumping-before-hydrolysis by electrometric studies. We suggest how key residues in helix 12, 13, and the exit channel loops affect ion selectivity and K + -activation due to a complex interplay of residues that are involved in subunit-subunit communication. Our findings not only explain ion selectivity in M-PPases but also why they display half-of-the-sites reactivity. Based on this, we propose, for the first time, a unified model for ion-pumping, hydrolysis, and energy coupling in all M-PPases, including those that pump both Na + and H + .
Organizational Affiliation:
Astbury Centre for Structural and Molecular Biology, University of Leeds, LS2 9JT, Leeds, UK.