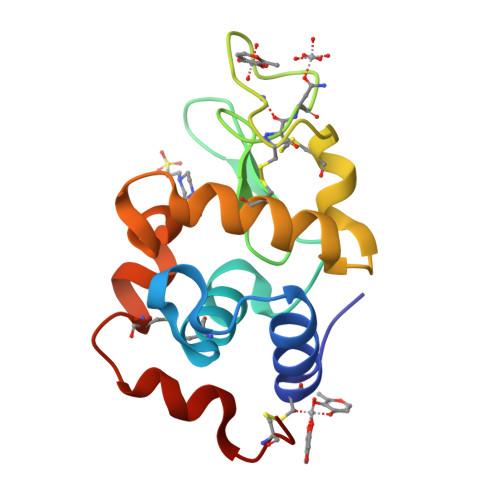
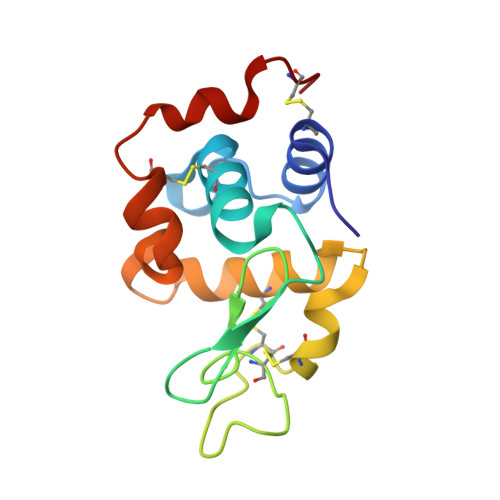
Multiple and Variable Binding of Pharmacologically Active Bis(maltolato)oxidovanadium(IV) to Lysozyme.
Ferraro, G., Paolillo, M., Sciortino, G., Garribba, E., Merlino, A.(2022) Inorg Chem 61: 16458-16467
- PubMed: 36205235
- DOI: https://doi.org/10.1021/acs.inorgchem.2c02690
- Primary Citation of Related Structures:
8AJ3, 8AJ4, 8AJ5 - PubMed Abstract:
The interaction with proteins of metal-based drugs plays a crucial role in their transport, mechanism, and activity. For an active ML n complex, where L is the organic carrier, various binding modes (covalent and non-covalent, single or multiple) may occur and several metal moieties (M, ML, ML 2 , etc.) may interact with proteins. In this study, we have evaluated the interaction of [V IV O(malt) 2 ] (bis(maltolato)oxidovanadium(IV) or BMOV, where malt = maltolato, i.e., the common name for 3-hydroxy-2-methyl-4 H -pyran-4-onato) with the model protein hen egg white lysozyme (HEWL) by electrospray ionization mass spectrometry, electron paramagnetic resonance, and X-ray crystallography. The multiple binding of different V-containing isomers and enantiomers to different sites of HEWL is observed. The data indicate both non-covalent binding of cis -[VO(malt) 2 (H 2 O)] and [VO(malt)(H 2 O) 3 ] + and covalent binding of [VO(H 2 O) 3-4 ] 2+ and cis -[VO(malt) 2 ] and other V-containing fragments to the side chains of Glu35, Asp48, Asn65, Asp87, and Asp119 and to the C-terminal carboxylate. Our results suggest that the multiple and variable interactions of potential V IV OL 2 drugs with proteins can help to better understand their solution chemistry and contribute to define the molecular basis of the mechanism of action of these intriguing molecules.
Organizational Affiliation:
Department of Chemical Sciences, University of Naples Federico II, Complesso Universitario di Monte Sant'Angelo, Via Cintia, I-80126Napoli, Italy.