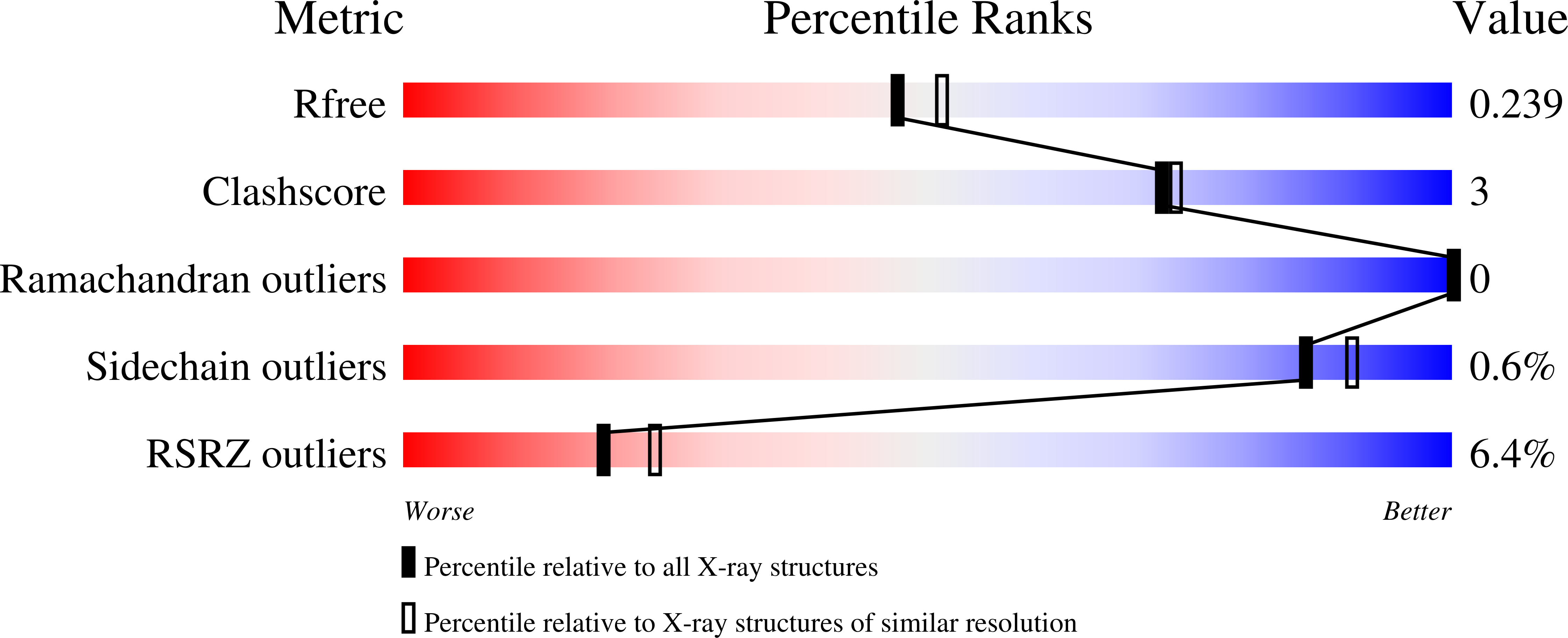
Decrypting the programming of beta-methylation in virginiamycin M biosynthesis.
Collin, S., Cox, R.J., Paris, C., Jacob, C., Chagot, B., Weissman, K.J., Gruez, A.(2023) Nat Commun 14: 1327-1327
- PubMed: 36899003
- DOI: https://doi.org/10.1038/s41467-023-36974-3
- Primary Citation of Related Structures:
8A7Z, 8AHQ, 8AHZ, 8AIG, 8ALL - PubMed Abstract:
During biosynthesis by multi-modular trans-AT polyketide synthases, polyketide structural space can be expanded by conversion of initially-formed electrophilic β-ketones into β-alkyl groups. These multi-step transformations are catalysed by 3-hydroxy-3-methylgluratryl synthase cassettes of enzymes. While mechanistic aspects of these reactions have been delineated, little information is available concerning how the cassettes select the specific polyketide intermediate(s) to target. Here we use integrative structural biology to identify the basis for substrate choice in module 5 of the virginiamycin M trans-AT polyketide synthase. Additionally, we show in vitro that module 7, at minimum, is a potential additional site for β-methylation. Indeed, analysis by HPLC-MS coupled with isotopic labelling and pathway inactivation identifies a metabolite bearing a second β-methyl at the expected position. Collectively, our results demonstrate that several control mechanisms acting in concert underpin β-branching programming. Furthermore, variations in this control - whether natural or by design - open up avenues for diversifying polyketide structures towards high-value derivatives.
Organizational Affiliation:
Université de Lorraine, CNRS, IMoPA, F-54000, Nancy, France.