Structural basis of alpha 1A -adrenergic receptor activation and recognition by an extracellular nanobody.
Toyoda, Y., Zhu, A., Kong, F., Shan, S., Zhao, J., Wang, N., Sun, X., Zhang, L., Yan, C., Kobilka, B.K., Liu, X.(2023) Nat Commun 14: 3655-3655
- PubMed: 37339967
- DOI: https://doi.org/10.1038/s41467-023-39310-x
- Primary Citation of Related Structures:
7YM8, 7YMH, 7YMJ - PubMed Abstract:
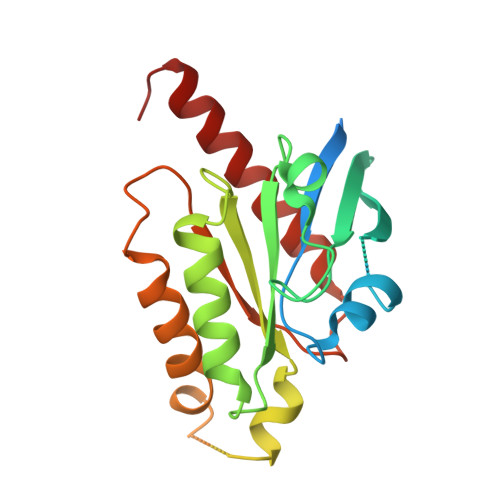
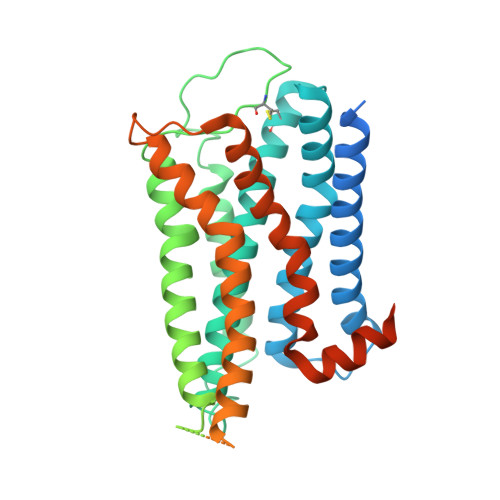
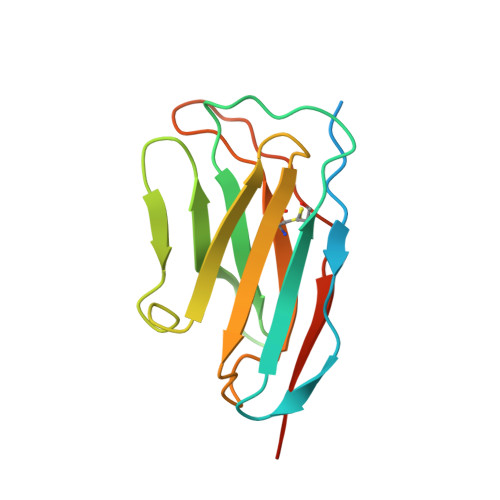
The α 1A- adrenergic receptor (α 1A AR) belongs to the family of G protein-coupled receptors that respond to adrenaline and noradrenaline. α 1A AR is involved in smooth muscle contraction and cognitive function. Here, we present three cryo-electron microscopy structures of human α 1A AR bound to the endogenous agonist noradrenaline, its selective agonist oxymetazoline, and the antagonist tamsulosin, with resolutions range from 2.9 Å to 3.5 Å. Our active and inactive α 1A AR structures reveal the activation mechanism and distinct ligand binding modes for noradrenaline compared with other adrenergic receptor subtypes. In addition, we identified a nanobody that preferentially binds to the extracellular vestibule of α 1A AR when bound to the selective agonist oxymetazoline. These results should facilitate the design of more selective therapeutic drugs targeting both orthosteric and allosteric sites in this receptor family.
- School of Medicine, Tsinghua University, Beijing, 100084, China. toyoda.yosuke.2r@kyoto-u.ac.jp.
Organizational Affiliation: