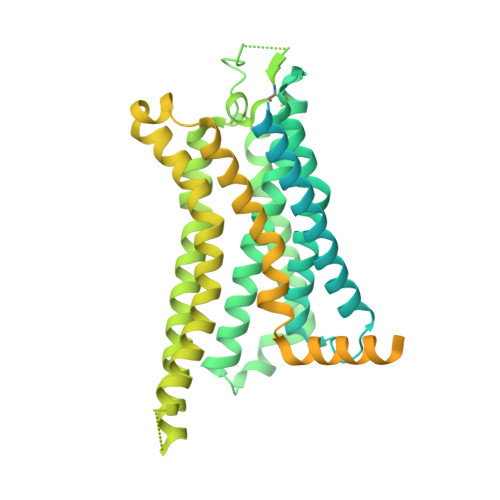
Cryo-EM structure of the human adenosine A 2B receptor-G s signaling complex.
Chen, Y., Zhang, J., Weng, Y., Xu, Y., Lu, W., Liu, W., Liu, M., Hua, T., Song, G.(2022) Sci Adv 8: eadd3709-eadd3709
- PubMed: 36563137
- DOI: https://doi.org/10.1126/sciadv.add3709
- Primary Citation of Related Structures:
7XY6, 7XY7 - PubMed Abstract:
The human adenosine A 2B receptor (A 2B R) is a class A G protein-coupled receptor that is involved in several major physiological and pathological processes throughout the body. A 2B R recognizes its ligands adenosine and NECA with relatively low affinity, but the detailed mechanism for its ligand recognition and signaling is still elusive. Here, we present two structures determined by cryo-electron microscopy of A 2B R bound to its agonists NECA and BAY60-6583, each coupled to an engineered G s protein. The structures reveal conserved orthosteric binding pockets with subtle differences, whereas the selectivity or specificity can mainly be attributed to regions extended from the orthosteric pocket. We also found that BAY60-6583 occupies a secondary pocket, where residues V250 6.51 and N273 7.36 were two key determinants for its selectivity against A 2B R. This study offers a better understanding of ligand selectivity for the adenosine receptor family and provides a structural template for further development of A 2B R ligands for related diseases.
- Shanghai Frontiers Science Center of Genome Editing and Cell Therapy, Shanghai Key Laboratory of Regulatory Biology, Institute of Biomedical Sciences and School of Life Sciences, East China Normal University, Shanghai 200241, China.
Organizational Affiliation: