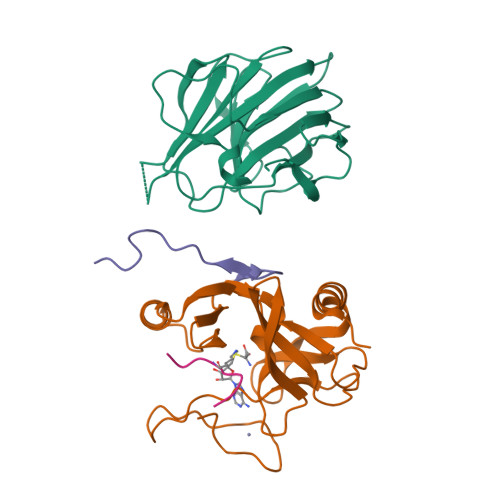
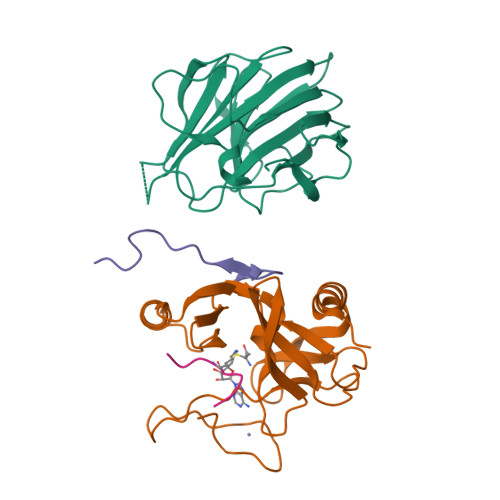
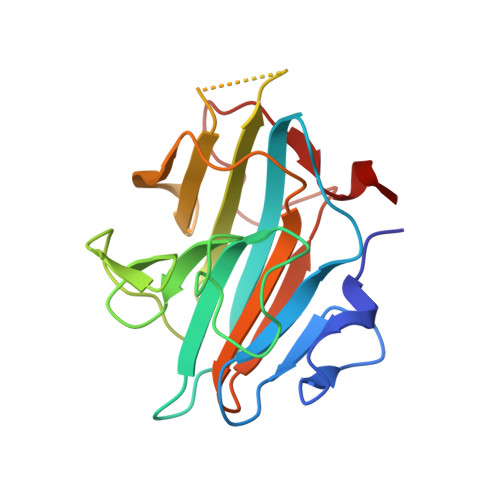
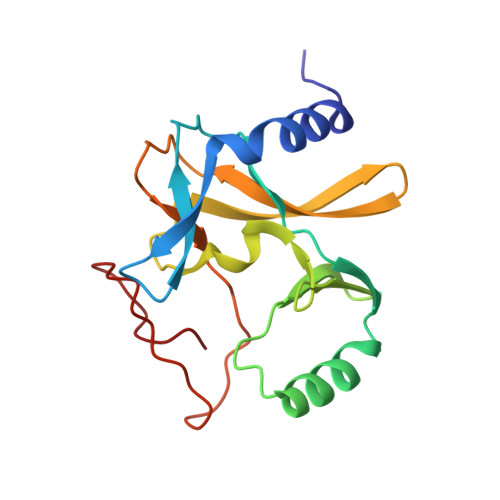
Structural basis for product specificities of MLL family methyltransferases.
Li, Y., Zhao, L., Zhang, Y., Wu, P., Xu, Y., Mencius, J., Zheng, Y., Wang, X., Xu, W., Huang, N., Ye, X., Lei, M., Shi, P., Tian, C., Peng, C., Li, G., Liu, Z., Quan, S., Chen, Y.(2022) Mol Cell 82: 3810-3825.e8
- PubMed: 36108631
- DOI: https://doi.org/10.1016/j.molcel.2022.08.022
- Primary Citation of Related Structures:
7W67, 7W6A, 7W6I, 7W6J, 7W6L - PubMed Abstract:
Human mixed-lineage leukemia (MLL) family methyltransferases methylate histone H3 lysine 4 to different methylation states (me1/me2/me3) with distinct functional outputs, but the mechanism underlying the different product specificities of MLL proteins remains unclear. Here, we develop methodologies to quantitatively measure the methylation rate difference between mono-, di-, and tri-methylation steps and demonstrate that MLL proteins possess distinct product specificities in the context of the minimum MLL-RBBP5-ASH2L complex. Comparative structural analyses of MLL complexes by X-ray crystal structures, fluorine-19 nuclear magnetic resonance, and molecular dynamics simulations reveal that the dynamics of two conserved tyrosine residues at the "F/Y (phenylalanine/tyrosine) switch" positions fine-tune the product specificity. The variation in the intramolecular interaction between SET-N and SET-C affects the F/Y switch dynamics, thus determining the product specificities of MLL proteins. These results indicate a modified F/Y switch rule applicable for most SET domain methyltransferases and implicate the functional divergence of MLL proteins.
Organizational Affiliation:
State Key Laboratory of Molecular Biology, National Center for Protein Science Shanghai, Shanghai Institute of Biochemistry and Cell Biology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China; State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai Collaborative Innovation Center for Biomanufacturing, Shanghai 200237, China.