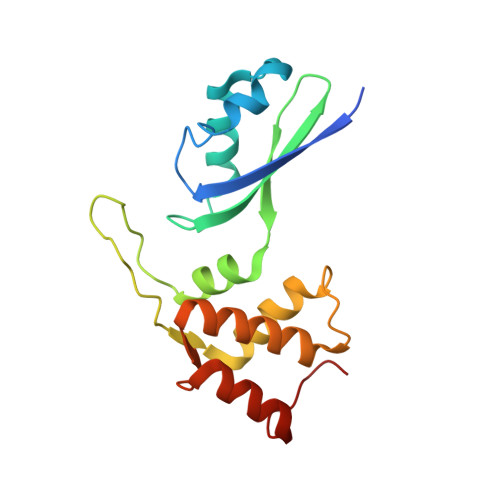
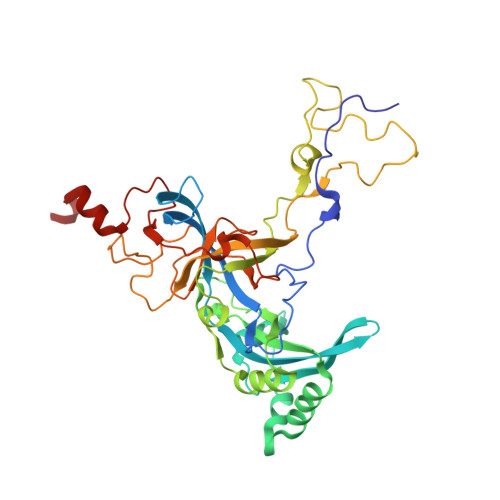
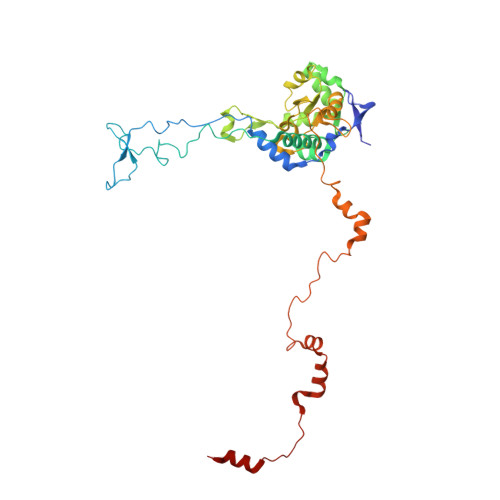
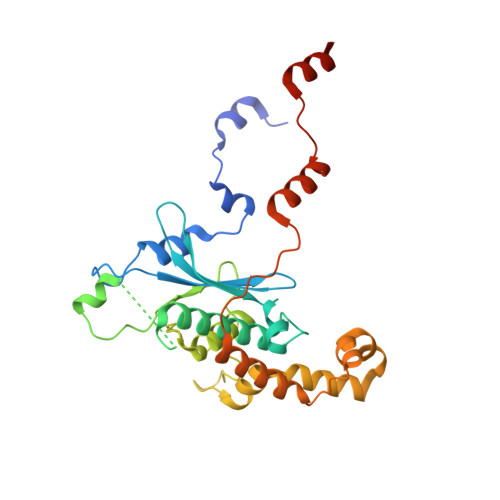
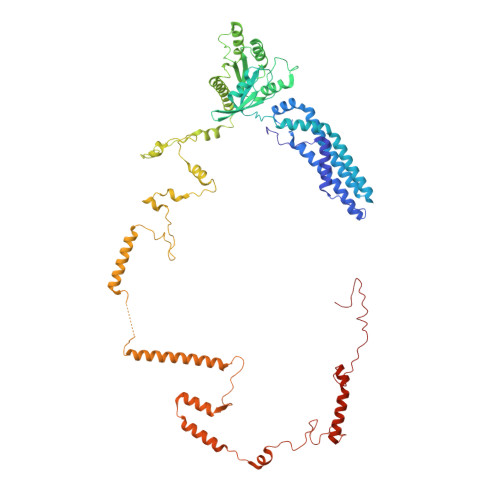
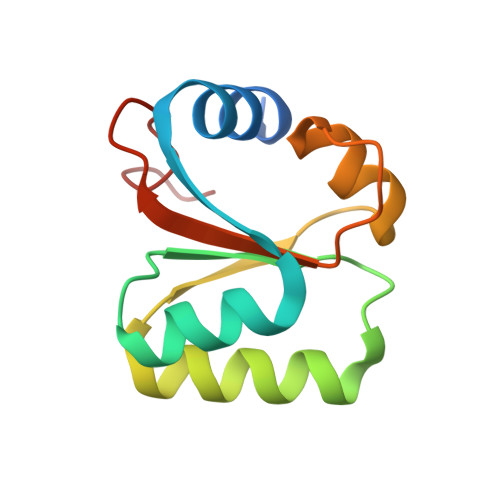
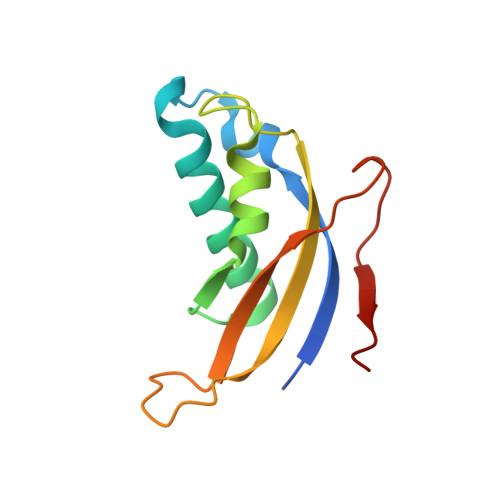
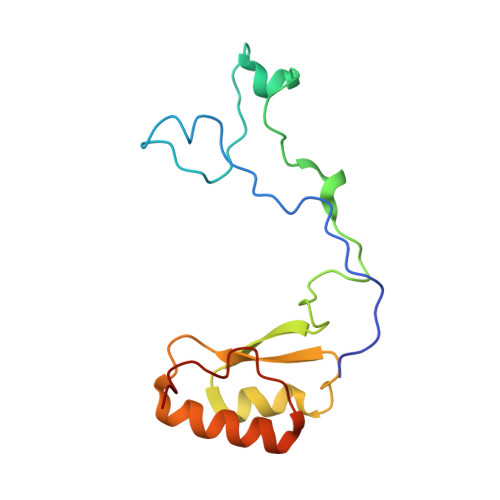
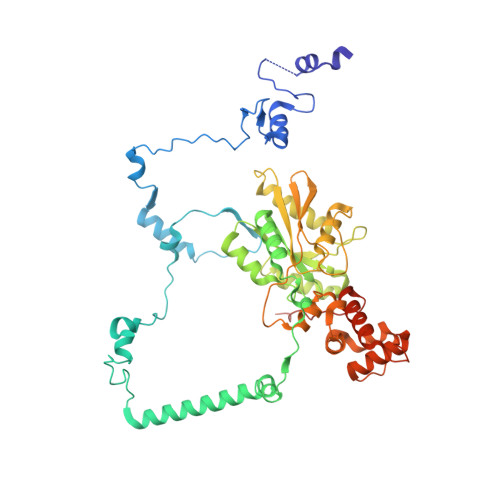
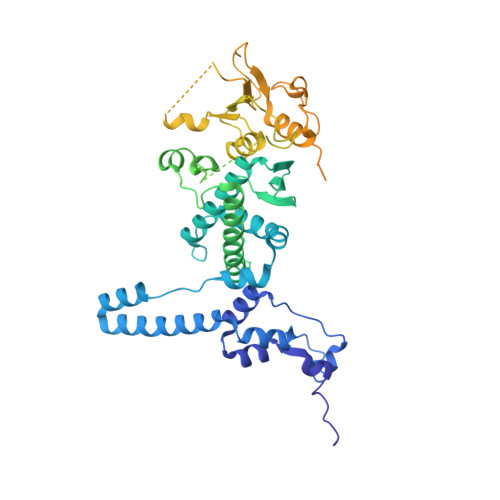
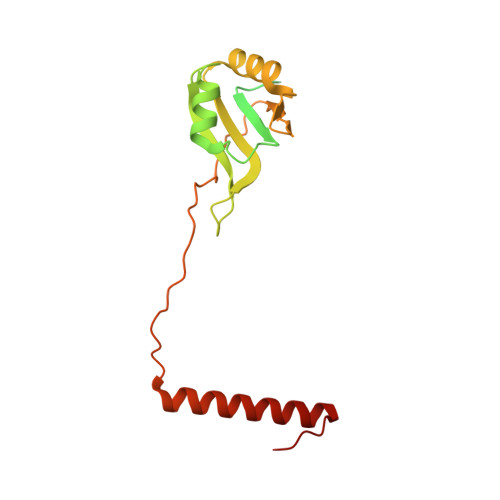
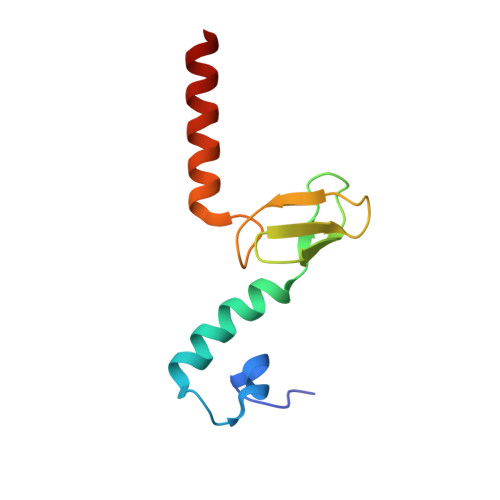
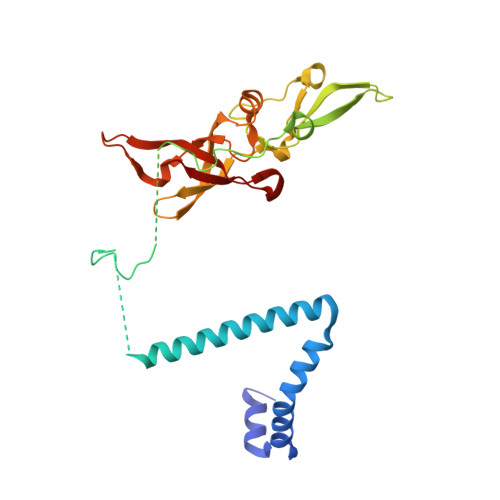
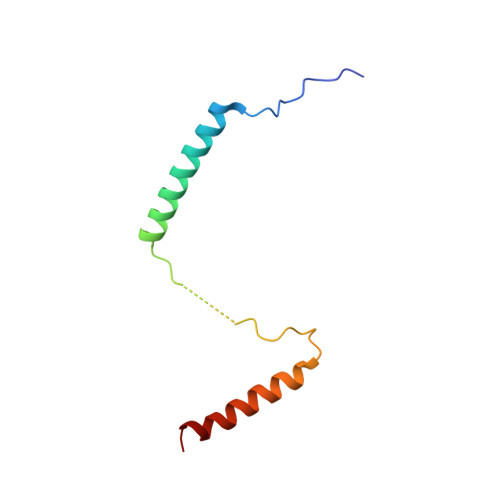
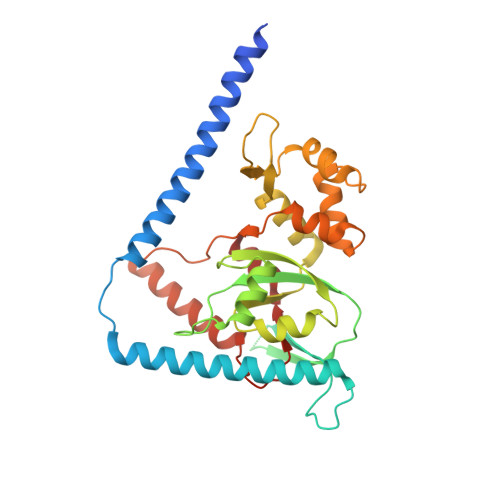
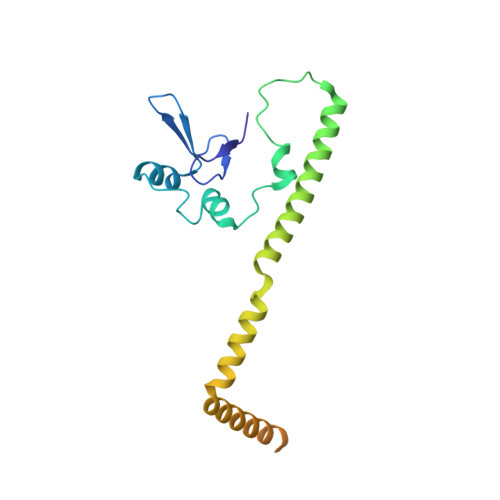
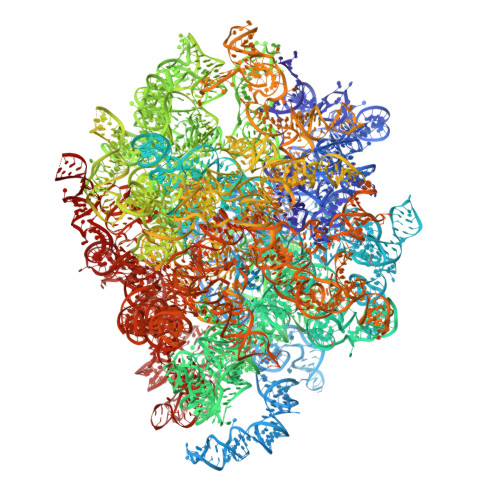
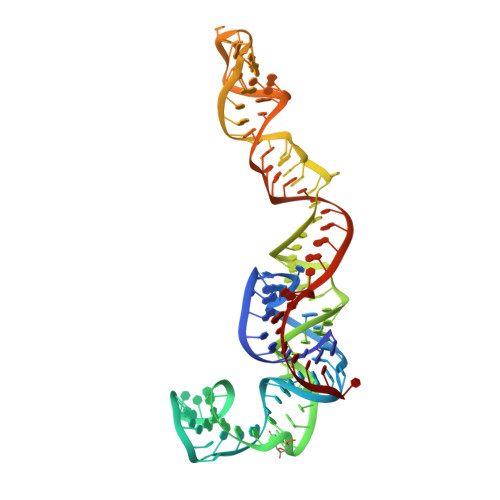
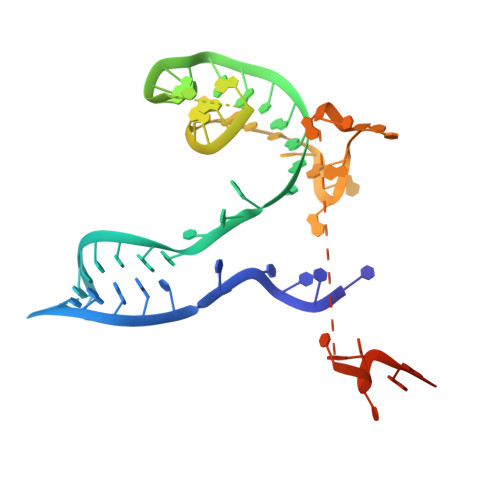
rRNA methylation by Spb1 regulates the GTPase activity of Nog2 during 60S ribosomal subunit assembly.
Sekulski, K., Cruz, V.E., Weirich, C.S., Erzberger, J.P.(2023) Nat Commun 14: 1207-1207
- PubMed: 36864048
- DOI: https://doi.org/10.1038/s41467-023-36867-5
- Primary Citation of Related Structures:
7UOO, 7UQB, 7UQZ, 7UUI, 7V08 - PubMed Abstract:
Biogenesis of the large ribosomal (60S) subunit involves the assembly of three rRNAs and 46 proteins, a process requiring approximately 70 ribosome biogenesis factors (RBFs) that bind and release the pre-60S at specific steps along the assembly pathway. The methyltransferase Spb1 and the K-loop GTPase Nog2 are essential RBFs that engage the rRNA A-loop during sequential steps in 60S maturation. Spb1 methylates the A-loop nucleotide G2922 and a catalytically deficient mutant strain (spb1 D52A ) has a severe 60S biogenesis defect. However, the assembly function of this modification is currently unknown. Here, we present cryo-EM reconstructions that reveal that unmethylated G2922 leads to the premature activation of Nog2 GTPase activity and capture a Nog2-GDP-AlF 4 - transition state structure that implicates the direct involvement of unmodified G2922 in Nog2 GTPase activation. Genetic suppressors and in vivo imaging indicate that premature GTP hydrolysis prevents the efficient binding of Nog2 to early nucleoplasmic 60S intermediates. We propose that G2922 methylation levels regulate Nog2 recruitment to the pre-60S near the nucleolar/nucleoplasmic phase boundary, forming a kinetic checkpoint to regulate 60S production. Our approach and findings provide a template to study the GTPase cycles and regulatory factor interactions of the other K-loop GTPases involved in ribosome assembly.
- Department of Biophysics, UT Southwestern Medical Center, 5323 Harry Hines Blvd., ND10.104B, Dallas, TX, 75390-8816, USA.
Organizational Affiliation: