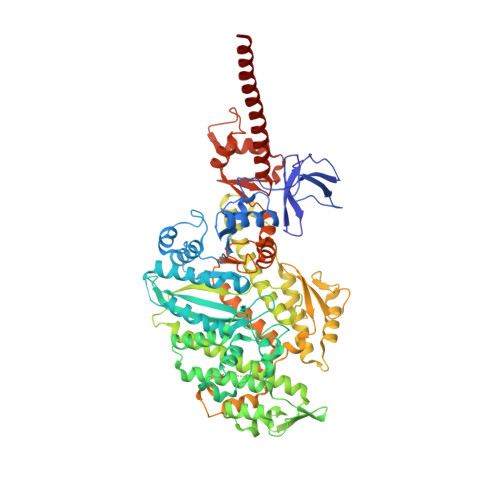
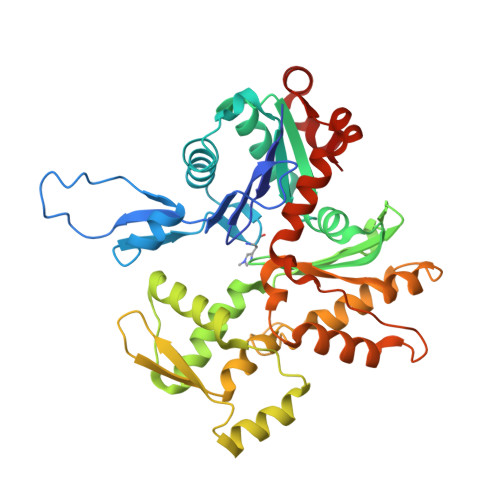
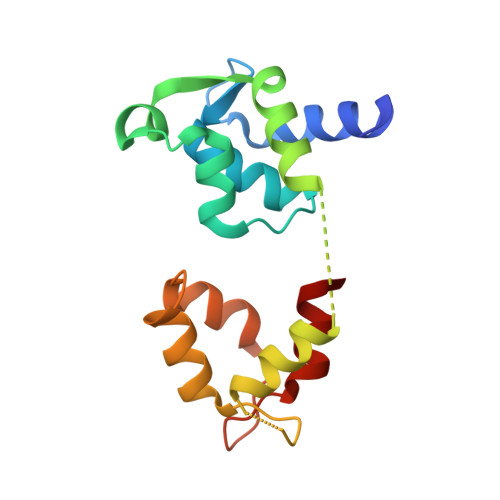
High-resolution structures of the actomyosin-V complex in three nucleotide states provide insights into the force generation mechanism.
Pospich, S., Sweeney, H.L., Houdusse, A., Raunser, S.(2021) Elife 10
- PubMed: 34812732
- DOI: https://doi.org/10.7554/eLife.73724
- Primary Citation of Related Structures:
7PLT, 7PLU, 7PLV, 7PLW, 7PLX, 7PLY, 7PLZ, 7PM0, 7PM1, 7PM2, 7PM3, 7PM5, 7PM6, 7PM7, 7PM8, 7PM9, 7PMA, 7PMB, 7PMC, 7PMD, 7PME, 7PMF, 7PMG, 7PMH, 7PMI, 7PMJ, 7PML - PubMed Abstract:
The molecular motor myosin undergoes a series of major structural transitions during its force-producing motor cycle. The underlying mechanism and its coupling to ATP hydrolysis and actin binding are only partially understood, mostly due to sparse structural data on actin-bound states of myosin. Here, we report 26 high-resolution cryo-EM structures of the actomyosin-V complex in the strong-ADP, rigor, and a previously unseen post-rigor transition state that binds the ATP analog AppNHp. The structures reveal a high flexibility of myosin in each state and provide valuable insights into the structural transitions of myosin-V upon ADP release and binding of AppNHp, as well as the actomyosin interface. In addition, they show how myosin is able to specifically alter the structure of F-actin.
- Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, Dortmund, Germany.
Organizational Affiliation: