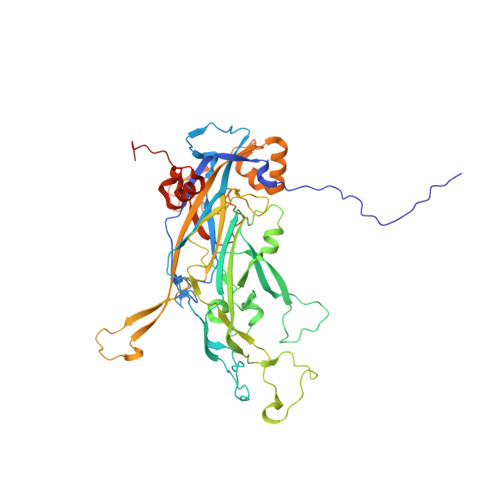
High resolution cryo EM analysis of HPV16 identifies minor structural protein L2 and describes capsid flexibility.
Goetschius, D.J., Hartmann, S.R., Subramanian, S., Bator, C.M., Christensen, N.D., Hafenstein, S.L.(2021) Sci Rep 11: 3498-3498
- PubMed: 33568731
- DOI: https://doi.org/10.1038/s41598-021-83076-5
- Primary Citation of Related Structures:
7KZF - PubMed Abstract:
Human papillomavirus (HPV) is a significant health burden and leading cause of virus-induced cancers. HPV is epitheliotropic and its replication is tightly associated with terminal keratinocyte differentiation making production and purification of high titer virus preparations for research problematic, therefore alternative HPV production methods have been developed for virological and structural studies. In this study we use HPV16 quasivirus, composed of HPV16 L1/L2 capsid proteins with a packaged cottontail rabbit papillomavirus genome. We have achieved the first high resolution, 3.1 Å, structure of HPV16 by using a local subvolume refinement approach. The high resolution enabled us to build L1 unambiguously and identify L2 protein strands. The L2 density is incorporated adjacent to conserved L1 residues on the interior of the capsid. Further interpretation with our own software for Icosahedral Subvolume Extraction and Correlated Classification revealed flexibility, on the whole-particle level through diameter analysis and local movement with inter-capsomer analysis. Inter-capsomer expansion or contraction, governed by the connecting arms, showed no bias in the magnitude or direction of capsomer movement. We propose that papillomavirus capsids are dynamic and capsomers move as rigid bodies connected by flexible linkers. The resulting virus structure will provide a framework for continuing biochemical, genetic and biophysical research for papillomaviruses. Furthermore, our approach has allowed insight into the resolution barrier that has previously been a limitation in papillomavirus structural studies.
- Department of Biochemistry and Molecular Biology, Penn State University, University Park, PA, 16802, USA.
Organizational Affiliation: