A quinolin-8-ol sub-millimolar inhibitor of UGGT, the ER glycoprotein folding quality control checkpoint.
Guay, K.P., Ibba, R., Kiappes, J.L., Vasiljevic, S., Boni, F., De Benedictis, M., Zeni, I., Le Cornu, J.D., Hensen, M., Chandran, A.V., Kantsadi, A.L., Caputo, A.T., Blanco Capurro, J.I., Bayo, Y., Hill, J.C., Hudson, K., Lia, A., Brun, J., Withers, S.G., Marti, M., Biasini, E., Santino, A., De Rosa, M., Milani, M., Modenutti, C.P., Hebert, D.N., Zitzmann, N., Roversi, P.(2023) iScience 26: 107919-107919
- PubMed: 37822503
- DOI: https://doi.org/10.1016/j.isci.2023.107919
- Primary Citation of Related Structures:
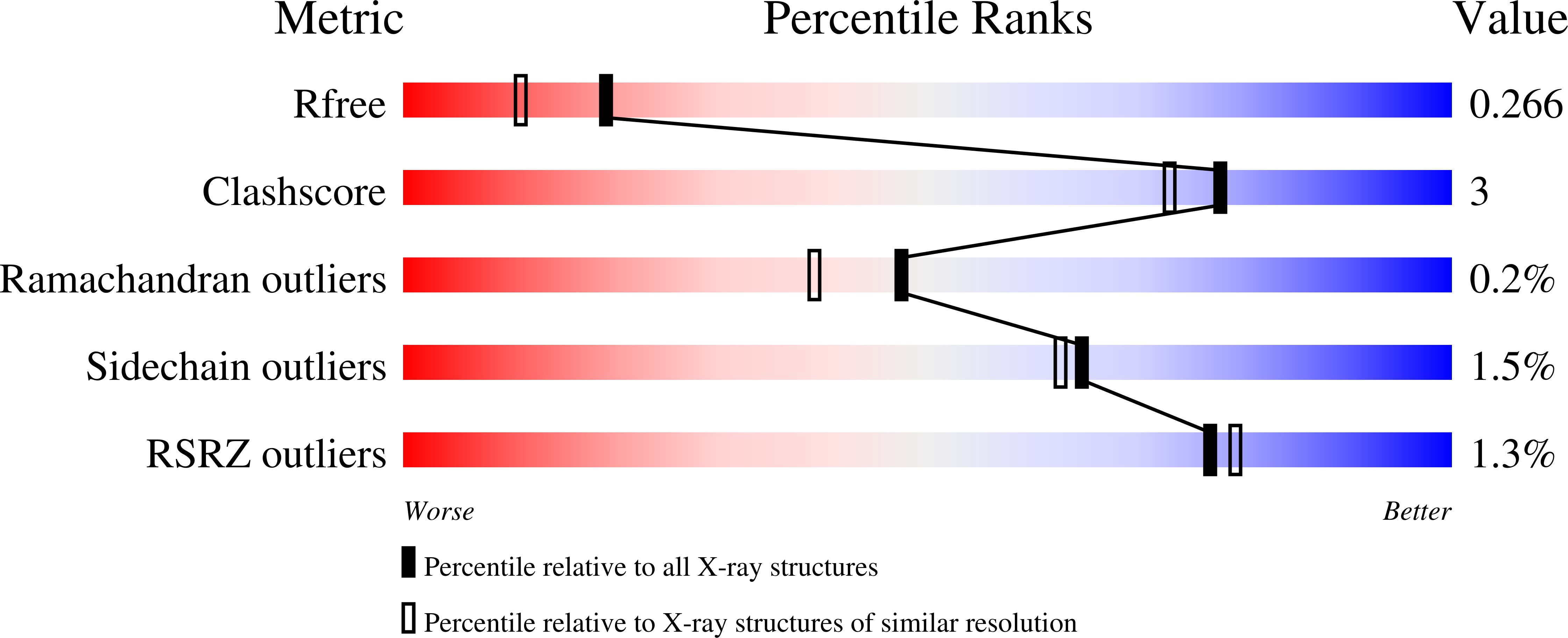
7ZKC, 7ZLL, 7ZLU - PubMed Abstract:
Misfolded glycoprotein recognition and endoplasmic reticulum (ER) retention are mediated by the ER glycoprotein folding quality control (ERQC) checkpoint enzyme, UDP-glucose glycoprotein glucosyltransferase (UGGT). UGGT modulation is a promising strategy for broad-spectrum antivirals, rescue-of-secretion therapy in rare disease caused by responsive mutations in glycoprotein genes, and many cancers, but to date no selective UGGT inhibitors are known. The small molecule 5-[(morpholin-4-yl)methyl]quinolin-8-ol (5M-8OH-Q) binds a Ct UGGT GT24 "WY" conserved surface motif conserved across UGGTs but not present in other GT24 family glycosyltransferases. 5M-8OH-Q has a 47 μM binding affinity for Ct UGGT GT24 in vitro as measured by ligand-enhanced fluorescence. In cellula , 5M-8OH-Q inhibits both human UGGT isoforms at concentrations higher than 750 μM. 5M-8OH-Q binding to Ct UGGT GT24 appears to be mutually exclusive to M5-9 glycan binding in an in vitro competition experiment. A medicinal program based on 5M-8OH-Q will yield the next generation of UGGT inhibitors.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, and Program in Molecular and Cellular Biology, University of Massachusetts, Amherst, MA, USA.