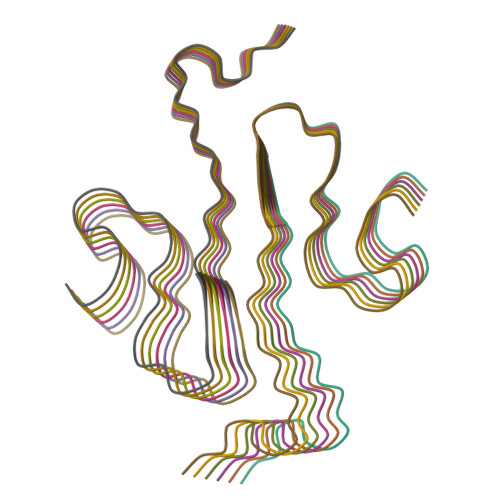
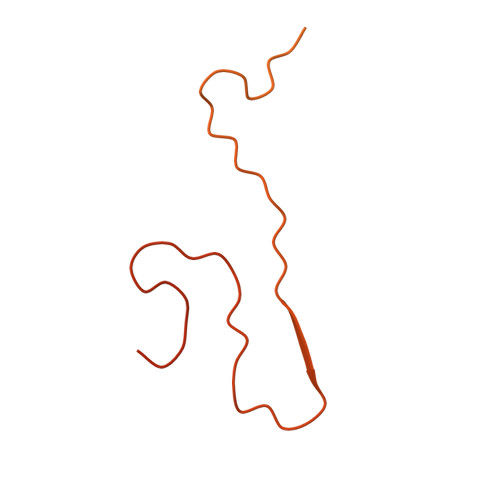
Cryo-EM Structure of the Full-length hnRNPA1 Amyloid Fibril.
Sharma, K., Banerjee, S., Savran, D., Rajes, C., Wiese, S., Girdhar, A., Schwierz, N., Lee, C., Shorter, J., Schmidt, M., Guo, L., Fandrich, M.(2023) J Mol Biology 435: 168211-168211
- PubMed: 37481159
- DOI: https://doi.org/10.1016/j.jmb.2023.168211
- Primary Citation of Related Structures:
7ZJ2 - PubMed Abstract:
Heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1) is a multifunctional RNA-binding protein that is associated with neurodegenerative diseases, such as amyotrophic lateral sclerosis and multisystem proteinopathy. In this study, we have used cryo-electron microscopy to investigate the three-dimensional structure of amyloid fibrils from full-length hnRNPA1 protein. We find that the fibril core is formed by a 45-residue segment of the prion-like low-complexity domain of the protein, whereas the remaining parts of the protein (275 residues) form a fuzzy coat around the fibril core. The fibril consists of two fibril protein stacks that are arranged into a pseudo-2 1 screw symmetry. The ordered core harbors several of the positions that are known to be affected by disease-associated mutations, but does not encompass the most aggregation-prone segments of the protein. These data indicate that the structures of amyloid fibrils from full-length proteins may be more complex than anticipated by current theories on protein misfolding.
Organizational Affiliation:
Institute of Protein Biochemistry, Ulm University, 89081 Ulm, Germany. Electronic address: kartikay.sharma@uni-ulm.de.