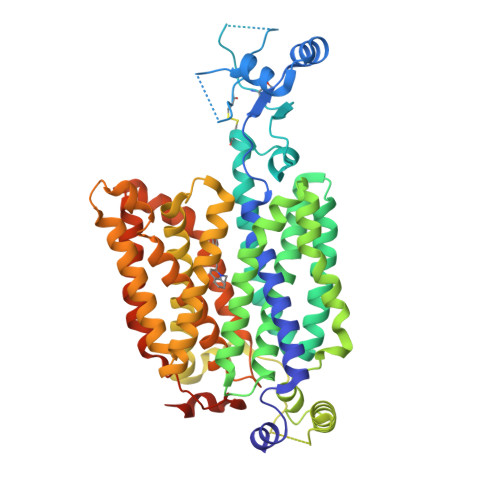
Structural basis of organic cation transporter-3 inhibition.
Khanppnavar, B., Maier, J., Herborg, F., Gradisch, R., Lazzarin, E., Luethi, D., Yang, J.W., Qi, C., Holy, M., Jantsch, K., Kudlacek, O., Schicker, K., Werge, T., Gether, U., Stockner, T., Korkhov, V.M., Sitte, H.H.(2022) Nat Commun 13: 6714-6714
- PubMed: 36344565
- DOI: https://doi.org/10.1038/s41467-022-34284-8
- Primary Citation of Related Structures:
7ZH0, 7ZH6, 7ZHA - PubMed Abstract:
Organic cation transporters (OCTs) facilitate the translocation of catecholamines, drugs and xenobiotics across the plasma membrane in various tissues throughout the human body. OCT3 plays a key role in low-affinity, high-capacity uptake of monoamines in most tissues including heart, brain and liver. Its deregulation plays a role in diseases. Despite its importance, the structural basis of OCT3 function and its inhibition has remained enigmatic. Here we describe the cryo-EM structure of human OCT3 at 3.2 Å resolution. Structures of OCT3 bound to two inhibitors, corticosterone and decynium-22, define the ligand binding pocket and reveal common features of major facilitator transporter inhibitors. In addition, we relate the functional characteristics of an extensive collection of previously uncharacterized human genetic variants to structural features, thereby providing a basis for understanding the impact of OCT3 polymorphisms.
Organizational Affiliation:
Laboratory of Biomolecular Research, Paul Scherrer Institute, Villigen, Switzerland.