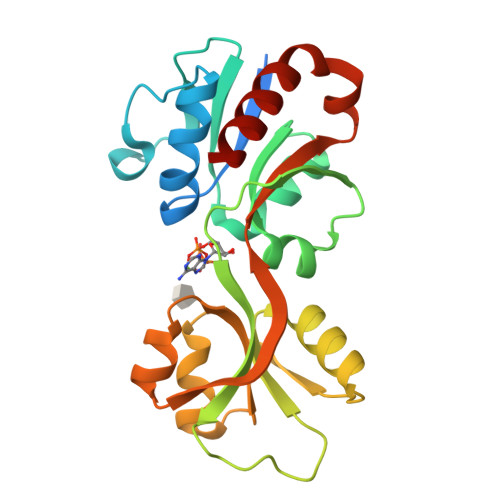
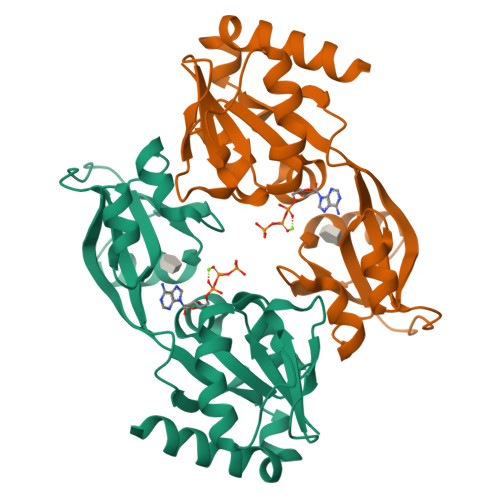
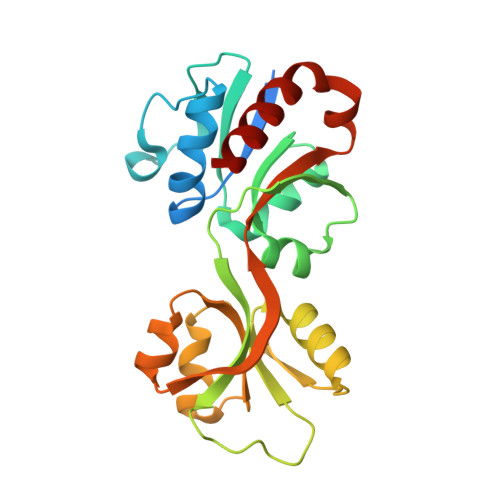
Allosteric rescue of catalytically impaired ATP phosphoribosyltransferase variants links protein dynamics to active-site electrostatic preorganisation.
Fisher, G., Corbella, M., Alphey, M.S., Nicholson, J., Read, B.J., Kamerlin, S.C.L., da Silva, R.G.(2022) Nat Commun 13: 7607-7607
- PubMed: 36494361
- DOI: https://doi.org/10.1038/s41467-022-34960-9
- Primary Citation of Related Structures:
7Z6R, 7Z8U - PubMed Abstract:
ATP phosphoribosyltransferase catalyses the first step of histidine biosynthesis and is controlled via a complex allosteric mechanism where the regulatory protein HisZ enhances catalysis by the catalytic protein HisG S while mediating allosteric inhibition by histidine. Activation by HisZ was proposed to position HisG S Arg56 to stabilise departure of the pyrophosphate leaving group. Here we report active-site mutants of HisG S with impaired reaction chemistry which can be allosterically restored by HisZ despite the HisZ:HisG S interface lying ~20 Å away from the active site. MD simulations indicate HisZ binding constrains the dynamics of HisG S to favour a preorganised active site where both Arg56 and Arg32 are poised to stabilise leaving-group departure in WT-HisG S . In the Arg56Ala-HisG S mutant, HisZ modulates Arg32 dynamics so that it can partially compensate for the absence of Arg56. These results illustrate how remote protein-protein interactions translate into catalytic resilience by restoring damaged electrostatic preorganisation at the active site.
Organizational Affiliation:
School of Biology, Biomedical Sciences Research Complex, University of St Andrews, St Andrews, KY16 9ST, UK.